One for the road! A study to assess the efficacy of single low-dose regimen of rasburicase in controlling hyperuricaemia in patients with tumour lysis syndrome due to haematological malignancies
Hamdy A Azim1,2, Sherif Ahmed Bahr2, Nermine Shawky Kamal2, Mohamed Adel Koura2, Rehab Tolba2, Heba Abdelmoneem Gad2, Ahmad Morsy1, Hossameldin Mohsen Attia2, Ibraheem Iskander2, Ahmed Hammad2, Mohammed Farouk Hemed2, Mohammed Fathy Abdallah2, Kareem Ahmed Sadek2 and Alaa Hamdi Taha2
1Department of Clinical Oncology, Faculty of Medicine, Cairo University, Cairo, Egypt
2Clinical Oncology and Bone Marrow Transplantation Unit, Manial Specialized University Hospital, Cairo University, Cairo, Egypt
Correspondence to: Hossameldin Mohsen Attia. Email: hossam.eldin.attia@gmail.com
Abstract
We conducted a retrospective audit of six patients with various haematological malignancies (two acute lymphoblastic leukaemia, one acute myeloid leukaemia, and three non-Hodgkin lymphoma); these patients were eligible to receive rasburicase, being at high risk of development of tumour lysis syndrome (TLS). They received a fixed single low-dose regimen of rasburicase (7.5 mg) mainly due to financial restriction, as patients were not supported by the National Health Service and did not have health insurance. We compared uric acid, creatinine levels, and electrolytes (i.e. phosphate, potassium, and calcium) before and after rasburicase administration and also assessed the need for renal replacement therapy after treatment.
All six patients had a significant reduction in uric acid levels on the first day, achieving a response rate of 100% (p = 0.008994); creatinine, phosphate, and potassium were reduced significantly as well, with the p values of 0.0439, 0.014326, and 0.002008, respectively; only one patient needed renal replacement therapy in the form of haemodialysis, due to concerns about hyperphosphataemia.
Financial difficulties faced either because patients lacked insurance or because of the restricted National Health Service budget in Egypt have resulted in the unavailability of certain modalities of treatment in cancer care and the need to consider more economic yet efficient approaches. Our experience suggests that a single low-dose rasburicase injection (7.5 mg) is an efficient and cost-effective method to control hyperuricaemia in patients with a high risk of developing TLS when compared with the more expensive and extended standard regimen and doses recommended.
Keywords: tumour lysis syndrome, hyperuricaemia, single dose of rasburicase, haemological malignancies
Introduction
Tumour lysis syndrome (TLS) is an overwhelming oncologic emergency that can result in serious consequences and requires prompt intervention. A great advance in the management of the condition has been achieved by adding rasburicase to the existing arsenal used to prevent and treat this condition, but the recommended regimen and doses can pose a financial challenge for patients with no insurance as well as being affected by the restricted budgets available for National Egyptian Health Services. With data and studies suggesting similar efficacy for abbreviated courses and doses of rasburicase in the management of TLS, there was a need to assess that strategy in a cohort of Egyptian patients to achieve a more economic approach while achieving reasonably comparable standards of efficiency, efficacy, and safety.
We conducted a retrospective case series reporting on six patients who received a fixed single low-dose regimen of rasburicase (7.5 mg) at high risk of developing TLS due to various haemotological malignancies. Uric acid and creatinine levels as well as the need for haemodialysis after rasburicase administration were analysed.
Our experience suggests that a single low-dose rasburicase injection (7.5 mg) seems to be an efficient and cost-effective method to control hyperuricaemia in patients at high risk of developing TLS.
Methods
Study objective
To evaluate single fixed dosing of 7.5 mg rasburicase in terms of controlling hyperuricaemia and subsequently serum creatinine in the treatment or prevention of TLS in patients with haematological malignancies, the primary end point is control of serum uric acid levels on days 1, 2, and 3 as well as on day 7 of follow-up to emphasise the maintenance of that control and the secondary end point is control of serum creatinine, serum phosphate, and serum potassium levels on days 1, 2, and 3 as well as on day 7 of follow-up.
Patients
During the period between May 2011 and July 2012, six patients within an age range of 18–50 years (mean = 35.5 years, SD = 10.49) at high risk of developing TLS due to various haematological malignancies, that is, non-Hodgkin lymphoma (NHL – 3), acute lymphoblastic leukaemia (ALL – 2), and acute myeloid leukaemia (AML – 1), were treated at the Clinical Oncology departments at Cairo University Hospitals by single low-dose rasburicase on seven occasions (one patient experienced high risk of TLS upon his initial presentation and upon disease relapse). Most patients bore a high tumour burden that was assumed by high total leukocytic counts (TLCs) that contributed to a high risk of TLS; two of seven patients had notable lymphadenopathy, but none had a bulky tumour mass (i.e., all masses noted were less than 10 cm in maximum diameter); none of the patients had a history or any signs suggestive of end-stage renal disease or chronic kidney disease. Upon presentation, all patients were hyperuricaemic, and five of the seven patients had creatinine levels > 1.5 × ULN with high risk of acute renal injury. The patients’ characteristics are shown in Table 1, and baseline laboratory findings in each patient are shown in Tables 2–6.
Patient evaluation
According to the current guidelines, patients with haematological malignancies at risk of development of TLS are evaluated at baseline for TLC, creatinine, and uric acid levels together with electrolytes, that is, potassium, calcium, and phosphorus. Follow-up lab work was done at least daily for the same parameters.
Table 1. Patients’ characteristics.
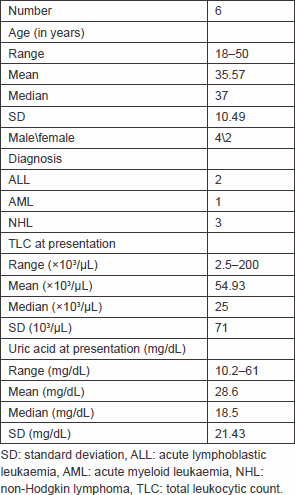
Table 2. Baseline laboratory findings before rasburicase administration.
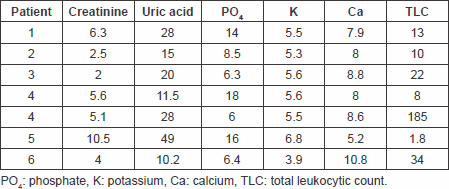
Table 3. Laboratory findings on day 1 follow-up.
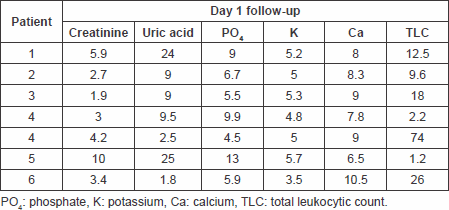
Table 4. Laboratory findings on day 2 follow-up.
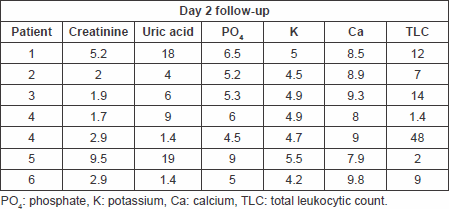
Table 5. Laboratory findings on day 3 follow-up.
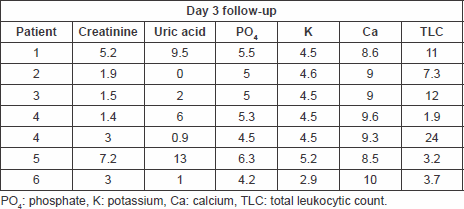
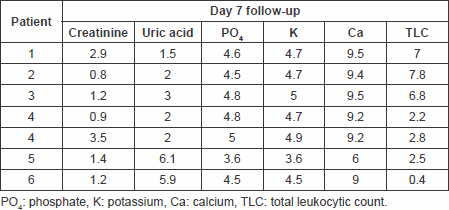
Table 6. Laboratory findings on day 7 follow-up.
Statistical analysis
All patients were included in the efficacy and safety analyses; response was defined as decline of serum uric acid levels below the threshold for hyperuricaemia and maintenance of this decline through days 1–7 of therapy. The improvement of renal functions was assessed by following the decline of serum creatinine levels and maintenance of this decline throughout days 1–7 of therapy; the decline in serum phosphate and serum potassium was also assessed throughout the 7 days of follow-up. Pre-treatment and post-treatment serum uric acid, serum creatinine, serum phosphate, and serum potassium levels were compared using a paired t test using Microsoft Excel software.
Adverse events were graded according to the World Health Organization toxicity criteria.
Treatment modalities
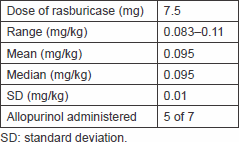
A total of six patients received rasburicase as a 30-minute intravenous infusion, at a fixed dose of 7.5 mg, in 50 mL preservative-free sodium chloride (range 0.083–0.11 mg/kg, mean 0.095 mg/kg, SD 0.01) on seven occasions (one patient experienced high risk of TLS upon his initial presentation and upon disease relapse). Patients only received treatment with rasburicase once with no subsequent injections planned unless clinically indicated, and none more were needed to any of the patients; all patients received intravenous hydration at 3–4 L/day monitored by their jugular venous pressure, and five of seven patients were treated with concomitant allopurinol at a dose of 300 mg orally twice daily, as shown in Table 7.
Table 7. Treatment modalities.
This study has been conducted in line with local regulations at the University of Cairo Hospital’s oncology department and after being approved by the institutional review board.
Results
Efficacy
The primary end point of a significant decrease in plasma uric acid levels on the first day following treatment with fixed single low dose of rasburicase was met with a p value of 0.008994 with a sustained response maintained through days 2 and 3 of follow-up. The response rate reached 100% as all patients’ uric acid levels normalised at day 4 of therapy. The significant reduction of uric acid levels at day 7 of therapy with no need for further treatment suggests the efficacy of the single-low dose approach.
Only one patient needed to undergo haemodialysis due to high phosphate levels rather than uric acid that were reduced, and there was no need to postpone chemotherapy or reduce chemotherapy doses in this patient. A significant reduction in plasma creatinine levels on the first day after treatment with a p value of 0.0439, which showed further decline in all patients on days 2 (p = 0.01299781) and 3 (p = 0.007507) of treatment up to day 7 (p = 0.018375), reflects a decreased incidence of acute renal failure requiring haemodialysis for management, thus meeting this secondary end point.
Secondary end points of significant reduction in serum phosphate and serum potassium levels on day 1 of follow-up post-treatment have also been met with p values of 0.014326 and 0.002008, respectively, a response that was also maintained throughout days 2–7 of follow-up.
Safety
All patients received treatment with good tolerability, and no grade 3 or 4 adverse events were recorded that were attributable to rasburicase administration. No cases of allergic reactions or haemolytic anaemia were recorded.
This study was conducted in line with local regulations at the University of Cairo Hospital’s oncology department and after being approved by the institutional review board.
Discussion
TLS is an oncologic emergency that can occur due to any type of neoplasm; it is caused by a massive lysis of tumour cells disturbing renal function, electrolytes, and haemodynamics and requiring immediate intervention.
Uric acid nephropathy due to mechanical obstruction by uric acid crystals in the renal tubules is the major cause of acute renal failure in the setting of TLS enhanced by high acidity and high concentration in the renal tubular fluid. Renal medullary haemoconcentration and decreased tubular flow rate also contribute to crystallisation [1].
Acute nephrocalcinosis due to calcium phosphate crystal precipitation, which may occur in other tissues, develops in the setting of hyperphosphataemia and is exacerbated by overzealous iatrogenic alkalinisation, because calcium phosphate, unlike uric acid, becomes less soluble at an alkaline pH.
TLS is highly associated with rapidly proliferating and bulky tumours compared with tumours of small volume or small tumour burden; Hande and Garrow showed in 1993 an incidence of TLS of 42% in a cohort of 102 patients with high-grade NHL most commonly in ALL and high-grade NHL, in particular Burkitt’s lymphoma. Other haematological malignancies that have a lower incidence of TLS include chronic lymphocytic leukaemia, AML, and plasma cell disorders including multiple myeloma and isolated plasmacytomas. In addition, TLS has been reported with other haematological malignancies including low-grade and intermediate-grade NHL, Hodgkin’s disease, chronic myeloid leukaemia (CML) in blast crisis, and myeloproliferative disorders [2].
In the context of treating patients presenting with high-risk features of developing TLS including hyperuricaemia due to highly proliferative neoplasms, especially haematological malignancies; an alternative to inhibiting uric acid formation by inhibiting xanthine oxidase with allopurinol is to promote the catabolism of uric acid to allantoin by urate oxidase. In comparison with uric acid, allantoin is five to ten times more soluble in urine [3], also avoiding accumulation of xanthine and hypoxanthine, which demonstrate poor water solubility and can worsen renal function [4]. Urate oxidase is an endogenous enzyme commonly found in many mammalian species but not in humans, secondary to a nonsense mutation in the coding region during hominoid evolution [5]. A non-recombinant urate oxidase, extracted from Aspergillus flavus, has been demonstrated to reduce uric acid levels in patients at risk for TLS and has been available in France since 1975 and in Italy since 1984 [6–8]. Recently, the gene coding for the urate oxidase was isolated as a cDNA clone from the A. flavus and expressed in the yeast Saccharomyces cerevisiae to yield large quantities of the pure recombinant form of urate oxidase (rasburicase) [9]. Pui et al initially reported the prophylactic use of rasburicase (15–20 mg/kg i.v. q.i.d. for 5–7 d) in 66 children with haematological malignancies at risk for TLS who presented with normouricaemic levels (uric acid <476 Lmol/L) [10]. Pui et al demonstrated a significant reduction of the median uric acid level of 256–30 Lmol/L (p < 0001) within 4 hours [10].
The phase III study results published by Cortes et al demonstrated efficacy and safety of rasburicase alone or followed by allopurinol compared with allopurinol alone in controlling plasma uric acid in adults at risk for TLS [11]; the results of the GRAAL1 (Grouped’Etude des Lymphomes de l’Adulte Trial on Rasburicase Activity in Adult Lymphoma) study have reached similar results before while assessing patients with aggressive NHL during induction treatment [12].
However, optimal dosage and timing of rasburicase should be discussed. Although the European Medicines Agency and the US Food and Drug Administration recommend a dosing range of 0.15 to 0.2 mg/kg/d for 5 days for rasburicase, several studies have demonstrated that a shorter treatment period or a lower dosage might have similar efficacy and might be cost saving [13–25]; one is a report published by Ho et al who demonstrated the efficacy of abbreviated doses of rasburicase in patients with high risk of developing TLS [23].
Another report by McBride et al concluded that the efficacy of all single fixed doses and weight-based dosing strategies evaluated in the study (3, 6, and 7.5 mg) appear to be comparable in normalising plasma uric acid levels within 24 hours of rasburicase administration, and although the use of a 3-mg rasburicase dose may be the most cost-effective treatment strategy in managing hyperuricaemia secondary to TLS, the 6-mg dose resulted in lower sustained uric acid levels after rasburicase administration [24].
Rasburicase is contraindicated in pregnancy and in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Conclusion
In this case series, we report on the efficacious use of single low-dose rasburicase injection in the control of serum uric acid levels in six patients with haematological malignancies at high risk of developing TLS all through seven days of follow-up post-treatment, significant reduction of uric acid levels (p = 0.008994, p = 0.003976, p = 0.00166, and p = 0.003399) at days 1, 2, 3, and 7 of therapy, respectively, with a response rate reaching 100% in all patients.
In addition, other biologic parameters involved in TLS were also controlled, demonstrating once again that the dramatic reduction of uric acid levels is the most important parameter for the prevention of TLS.
Renal function was almost preserved with significant reduction in serum creatinine levels (p = 0.043906828, p = 0.01299781, p = 0.007507, and p = 0.018375) on days 1, 2, 3, and 7, respectively, only one patient requiring haemodialysis for acute renal failure due to high levels of serum phosphate rather than high serum uric acid levels, which is of paramount importance in the management of these patients and for the prevention of many complications of chemotherapy. Rasburicase was excellently tolerated as well.
There has been significant reduction of serum phosphate (p = 0.014326, p = 0.010934, p = 0.008864, and p = 0.010825) for days 1, 2, 3, and 7 respectively, and serum potassium levels (p = 0.002008, p = 0.006224, p = 0.0069915, and p = 0.0044933) for days 1, 2, 3, and 7, respectively, as well.
From an economic point of view, the promising single low-dose approach of rasburicase administration achieved satisfactory results with a great reduction of cost per patient (approximately 3000 LE for the single low-dose approach versus 6000–42000 LE for the standard approved regimen), which can result in better cost effectiveness achieved by physicians caring for such patients with no compromise of clinical efficacy.
The small number of our sample of patients is regarded as a limitation to our study and shows the need for a study on a larger scale to emphasise our results.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
1. Abu-Alfa AK and Younes A (2010) Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management Am J Kidney Dis 55(5 Suppl 3) S1–13 quiz S14–9 DOI: 10.1053/j.ajkd.2009.10.056 PMID: 20420966
2. Cairo MS and Bishop M (2004) Tumour lysis syndrome: new therapeutic strategies and classification Br J Haematol 127 3–11 DOI: 10.1111/j.1365-2141.2004.05094.x PMID: 15384972
3. Brogard JM, Coumaros D, Franckhauser J, Stahl A and Stahl J (1972) Enzymatic uricolysis: a study of the effect of a fungal urateoxydase Revue Europeenne D Etudes Cliniqueset Biologiques 17 890–5
4. Behl D, Hendrickson AW and Moynihan TJ (2010) Oncologic emergencies Crit Care Clin 26 181–205 DOI: 10.1016/j.ccc.2009.09.004
5. Yeldandi AV, Yeldandi V, Kumar S, Murthy CV, Wang XD, Alvares K, Rao MS and Reddy JK (1991) Molecular evolution of the urate oxidase-encoding gene in hominoid primates: nonsense mutations Gene 109 281–4 DOI: 10.1016/0378-1119(91)90622-I PMID: 1765273
6. Masera G, Jankovic M, Zurlo MG, Locasciulli A, Rossi MR, Uderzo C and Recchia M (1982) Urate-oxidase prophylaxis of uric acid-induced renal damage in childhood leukemia J Pediatr 100 152–5 DOI: 10.1016/S0022-3476(82)80259-X PMID: 6948943
7. Pui CH, Relling MV, Lascombes F, Harrison PL, Struxiano A, Mondesir JM, Ribeiro RC, Sandlund JT, Rivera GK, Evans WE and Mahmoud HH (1997) Urate oxidase in prevention andtreatment of hyperuricemia associated with lymphoid malignancies Leukemia 11 1813–6 DOI: 10.1038/sj.leu.2400850 PMID: 9369411
8. Patte C, Sakiroglu C, Ansoborlo S, Baruchel A, Plouvier E, Pacquement H and Babin-Boilletot A (2002) Urate-oxidase in the prevention and treatment of metabolic complications in patients with B-cell lymphoma and leukemia, treated in the Societe Francaised’Oncologie Pediatrique LMB89 protocol Ann Oncol 13 789–95 DOI: 10.1093/annonc/mdf134 PMID: 12075750
9. Legoux R, Delpech B, Dumont X, Guillemot JC, Ramond P, Shire D, Caput D, Ferrara P and Loison G (1992) Cloning and expression in Escherichia coli of the gene encoding Aspergillusflavusurate oxidase J Biol Chem 267 8565–70 PMID: 1339455
10. Pui CH, Mahmoud HH, Wiley JM, Woods GM, Leverger G, Camitta B, Hastings C, Blaney SM, Relling MV and Reaman GH (2001) Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients with leukemia or lymphoma J Clin Oncol 19 697–704 PMID: 11157020
11. Cortes et al (2010) Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone – results of a multicenter phase III study J Clin Oncol 28(27) 4207–13 DOI: 10.1200/JCO.2009.26.8896 PMID: 20713865
12. Coiffier et al (2003) Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin’s lymphoma: results of the GRAAL1 (Grouped’Etude des Lymphomes de l’Adulte Trial on Rasburicase Activity in Adult Lymphoma) study J Clin Oncol 21(23) 4402–6 DOI: 10.1200/JCO.2003.04.115 PMID: 14581437
13. Lee AC, Li CH, So KT, et al (2003) Treatment of impending lumorlysis with single-dose rasburicase Ann Pharmacother 37 1614–7 DOI: 10.1345/aph.1D111 PMID: 14565793
14. McDonnell AM, Lenz KL, Frei-Lahr DA, et al (2006) Single-dose rasburicase 6 mg in the management of tumor lysis syndrome in adults Pharmacotherapy 26 806–12 DOI: 10.1592/phco.26.6.806 PMID: 16716134
15. Liu CY, Sims-McCallum RP and Schiffer CA (2005) A single dose of rasburicase is sufficient for the treatment of hyperuricemia in patients receiving chemotherapy Leuk Res 29 463–5 DOI: 10.1016/j.leukres.2004.09.004 PMID: 15725482
16. Trifilio S, Gordon L, Singhal S, et al (2006) Reduced-dose rasburicase (recombinant xanthine oxidase) in adult cancer patients with hyperuricemia Bone Marrow Transplant 37 997–1001 DOI: 10.1038/sj.bmt.1705379 PMID: 16708061
17. Hutcherson DA, Gammon DC, Bhatt MS, et al (2006) Reduced-dose rasburicase in the treatment of adults with hyperuricemia associated with malignancy Pharmacotherapy 26 242–7 DOI: 10.1592/phco.26.2.242 PMID: 16466328
18. Hummel M, Reiter S, Adam K, et al (2008) Effective treatment and prophylaxis of hyperuricemia and impaired renal function in tumor lysis syndrome with low doses of rasburicase Eur J Haematol 80 331–6 DOI: 10.1111/j.1600-0609.2007.01013.x
19. Reeves DJ and Bestul DJ (2008) Evaluation of a single fixed dose of rasburicase 7.5 mg for the treatment of hyperuricemia in adults with cancer Pharmacotherapy 28 685–90 DOI: 10.1592/phco.28.6.685 PMID: 18503395
20. Campara M, Shord SS and Haaf CM (2009) Single-dose rasburicase for tumourlysis syndrome in adults: weight-based approach J Clin Pharm Ther 34 207–13 DOI: 10.1111/j.1365-2710.2008.00994.x PMID: 19250141
21. Giraldez M and Puto K (2010) A single, fixed dose of rasburicase (6 mg maximum) for treatment of tumor lysis syndrome in adults Eur J Haematol 85 177–9 PMID: 20394650
22. Trifilio SM, Pi J, Zook J, et al (2010) Effectiveness of a single 3-mg rasburicase dose for the management of hyperuricemia in patients with hematological malignancies Bone Marrow Transplant [epub ahead of print on September 6, 2010] PMID: 20818444
23. Ho VQ, Wetzstein GA, Patterson SG and Bradbury R (2006) Abbreviated rasburicase dosing for the prevention and treatment of hyperuricemia in adults at risk for tumor lysis syndrome Support Cancer Ther 3(3) 178–82 DOI: 10.3816/SCT.2006.n.016
24. McBride A, Lathon SC, Boehmer L, Augustin KM, Butler SK and Westervelt P (2013) Comparative evaluation of single fixed dosing and weight-based dosing of rasburicase for tumor lysis syndrome Pharmacotherapy 33(3) 295–303 DOI: 10.1002/phar.1198 PMID: 23456733
25. Firwana et al (2012) Tumor lysis syndrome: a systematic review of case series and case reports Postgrad Med 124(2) 92–101 DOI: 10.3810/pgm.2012.03.2540 PMID: 22437219






