Current landscape of BRAF-V600E metastatic CRC management in Latin America: an expert Latin American panel’s recommendations
Anelisa K Coutinho1, Yazmin Carolina Blanco Vazquez2, Markus Andret Cavalcante Gifoni3,4, Angela Marie Jansen5, Juan Manuel O’Connor6, Juan Carlos Samamé Pérez-Vargas7,8, Mariana Rico-Restrepo9, Gayatri Sanku5 and Guillermo Mendez10
1Clínica AMO, DASA, Salvador, Bahia, Brazil
2Centro Médico ABC Observatorio, Ciudad de Mexico 01120, Mexico
3Universidade Federal do Ceara, Fortaleza 60430-160, Brazil
4Oncologia D’Or, Fortaleza 60135-237, Brazil
5Americas Health Foundation, Washington, DC, USA
6Instituto Alexander Fleming, Buenos Aires, Argentina
7Clínica San Felipe, Lima, Peru
8Hospital Nacional Arzobispo Loayza, Lima 15072, Peru
9Americas Health Foundation, Bogota, Colombia
10Hospital de Gastroenterologia ‘Carlos Bonorino Udaondo’, Buenos Aires 1264, Argentina
ahttps://orcid.org/0000-0003-2988-0446
bhttps://orcid.org/0000-0003-1269-4567
chttps://orcid.org/0000-0003-2980-2421
dhttps://orcid.org/0000-0002-6975-5466
ehttps://orcid.org/0000-0001-7818-8670
fhttps://orcid.org/0000-0001-7234-0897
ghttps://orcid.org/0009-0002-8550-957X
Abstract
Colorectal cancer is the second leading cause of cancer death in Latin America (LA) with a projected 65.4% increase by 2040. Up to 10% of metastatic CRC (mCRC) patients in LA had an activating BRAF mutation. In clinical trials, targeted therapies for BRAF-V600E mutated mCRC have improved patient outcomes. However, in LA, BRAF-V600E testing and treatment of positive patients remains variable. To address this need, the Americas Health Foundation convened a meeting of LA experts on BRAF-V600E mCRC to develop treatment recommendations. The expert panel addressed the current landscape of BRAF-V600E mCRC testing, diagnosis and treatment in the region and identified significant limitations. Local guidelines, multidisciplinary boards, and tumor genotyping are among the recommendations. The panel also made first-line, second-line and surgery recommendations for patients after diagnosis.
Keywords: mCRC in Latin America, metastatic colorectal cancer, precision medicine, V600E-BRAF mCRC, V600E-BRAF-mutation
Correspondence to: Anelisa K Coutinho
Email: coutinhoanelisa@gmail.com
Published: 04/12/2024
Received: 10/06/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
BRAF-V600E mutated metastatic colorectal cancer (mCRC) (BRAF-V600E mCRC) impacts patients globally; Latin America (LA) has a 4%–12% prevalence. The v-RAF murine sarcoma viral oncogene homolog B1 protein (BRAF) is the primary kinase in the RAS/RAF/mitogen-activated protein kinas (MEK)/MAPK intracellular-signaling pathway regulating the cell cycle. The BRAF-V600E mutation causes constitutive kinase activation, uncontrolled cell division and neo-angiogenesis and metastases [1]. BRAF mutations are found in up to 10% of patients with mCRC and 95% are V600E [2]. While chemotherapeutics are available for patients with mCRC [3], patients with BRAF V600E-mutated mCRC have an 11–19 month median overall survival (OS), indicating a therapeutic gap in the efficacy of standard mCRC therapies. Recently, novel therapeutics have improved BRAF-mutated mCRC outcomes for patients globally. However, delayed regulatory approvals and affordability compromise treatment access in LA. This paper describes the landscape of BRAF-V600E mCRC therapy in LA and suggests ways to improve treatment and patient outcomes.
Methods
AHF used PubMed, MEDLINE and EMBASE to identify LA-based oncologists who have published in colorectal cancer (CRC), oncology and BRAF testing since. AHF used the following search terms: ‘BRAF,’ ‘BRAFV600,’ ‘molecular testing’ and ‘ CRC’ in combination with ‘ LA ‘ from 1 January 2017 to 10 June 2022. The identified articles were in English, Portuguese and Spanish. They met on 28, 29 November and 1 December 2022, to examine the landscape of diagnosing and treating BRAF-V600E CRC in LA and to create recommendations for optimum diagnosis and treatment. AHF assigned each panel member a question on BRAF-V600E CRC in LA. Individual panel members answered questions based on the AHF literature review, their reviews and personal knowledge. The panel reviewed and amended each answer during a 3-day meeting with many discussion rounds. Following the meeting, the panel evaluated and approved the final document.
Results
Epidemiology of mCRC
According to GLOBOCAN, CRC is the third most common cancer, and the second largest cause of death in LA and the Caribbean, with 186.5 cases and 86.5 deaths per 100,000 people. Alarmingly, LA CRC incidence is expected to rise by 65.4% by 2040 [1].
BRAF-V600E is seen in over 95% of BRAF-mutated mCRC patients and is female-associated, often right-sided and advanced, mucinous, with deficient mismatch repair (dMMR) and serrated adenoma pathway mutation [2, 4–6]. Among a cohort of 1,742 patients diagnosed with CRC in Argentina, Brazil, Chile, Mexico, Paraguay, Peru and Puerto Rico, the prevalence of BRAF-V600E mutation was 47.8% [7]. In a meta-analysis of BRAF-V600E mutation frequency across LA, variations in frequency were found by region (4.0%–12.2%), underscoring the importance of detecting and managing this form of mCRC in LA [8].
Understanding BRAF-mutational status in LA
BRAF-V600E mCRC cases are increasing in prevalence and mortality across LA. In Mexico, scientists discovered regional differences in BRAF-V600E tumors (Western Mexico 4%, Northeast Mexico 0% and Central Mexico 9.6%) [9, 10]. BRAF-V600E mCRC frequency has also been studied in Argentina (12.2%) [11], Brazil (6.5%, 8.7% and 6.6%) [12–14], Chile (9% and 12%) [15, 16] and Peru (9.9% and 10%) [17, 18]. Regionally, there are still gaps in the detection and mutational analysis of BRAF-V600E [19] (Figure 1).
To prescribe the optimal treatment, the RAS/BRAF-mutational status must be determined by either a tumor biopsy (primary or metastatic) or a less invasive liquid biopsy (LB) if no tumor material is available [20]. The advantages of liquid-based molecular profiling include studying intra- and inter-tumor genomic heterogeneity and performing tumor profiling without tissue.
Without local guidelines on screening, treating and monitoring BRAF-V600E mCRC, oncologists in LA often use international standards for testing and treatment decisions. The Argentine Association of Clinical Oncology recommends performing RAS, BRAF and MMR/MSI testing in patients with mCRC [22]. The Brazilian Gastrointestinal Tumors Group recommends having the same molecular profile in the metastatic setting [23]. In Chile, Mexico, Peru and Uruguay, local guidelines created by government entities or expert consensus follow international recommendations [22, 24–26]. BRAF molecular testing is generally required once patients are in the metastatic setting.
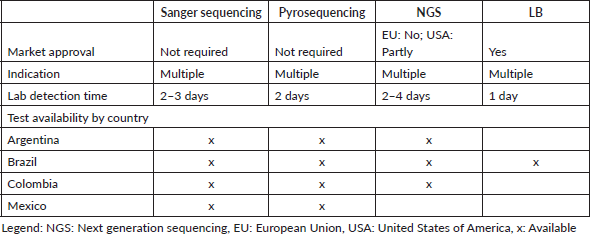
Access to tools for BRAF molecular testing is limited across LA. Real-time polymerase chain reaction (RT-PCR) and next-generation sequencing (NGS) are available, but only via clinical trials because of their high cost (Table 1). Despite the higher accuracy of RT-PCR, some local laboratories across LA still apply Sanger Sequencing, although the accuracy may be suboptimal. Pharmaceutical companies sponsor RAS and BRAF testing for anti-EGFR therapeutic management across LA to support patient access to precision medicine diagnostics.
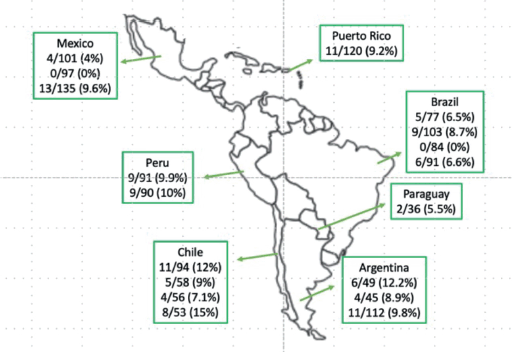
Figure 1. BRAF-V600E CRC prevalence in LA. Data taken from published studies [9–18].
Table 1. BRAF-V600E for mCRC testing in Argentina, Brazil, Colombia and Mexico.
Value of early BRAF-mutation status in mCRC in therapeutic decision-making and understanding its importance
Early BRAF status detection is critically important from a prognostic, predictive and therapeutic standpoint. Before starting first-line therapy in mCRC patients, international guidelines recommend BRAF, RAS and MSI testing.
BRAF-mutated tumors, particularly those harboring a V600E mutation, are associated with significantly poorer OS than BRAF-V600 wild-type tumors (median 10.4 versus 34.7 months), and a higher rate of peritoneal and distant lymph node metastasis [27]. Other randomised clinical trials also reported the negative impact of BRAF mutations [7, 28, 29]. Another advantage of identifying the BRAF status early is connected to MSI. If a BRAF mutation is found in dMMR/MSI tumors, Lynch syndrome can mostly be ruled out. Thus, determining BRAF status is of diagnostic and therapeutic relevance and helps differentiate somatic (sporadic) from germline (hereditary) dMMR [30–32].
RAF protein dimerization and sensitivity to BRAF and MEK inhibitors support the proposed molecular classification for BRAF mutations; Class I (including V600E) mutations have BRAF activity as monomers. Class II mutations are active only as dimers. Both are RAS-independent in activating the MAPK pathway. Class III BRAF mutations are not intrinsically active, requiring coexisting RAS mutations to activate the MAPK system. RAS (KRAS and NRAS) mutations in the RAS-RAF-MAPK and PI3K-AKT-mTOR signaling pathways are recognised as anti-EGFR resistant biomarkers [27]. The non-V600E-BRAF mutations represent less than 5% of BRAF mutations. They confer a similar prognosis as RAS/BRAF wild-type tumors. Some reports suggest that non-BRAF-V600E-mutated tumors might benefit from anti-EGFR therapy.
According to the European Society for Medical Oncology (ESMO) guidelines [20], BRAF-V600E characterization is vital for prognosis and treatment. First-line treatment includes chemotherapy with the classic scheme of FOLFOX or FOLFIRI plus a biologic, preferably anti-angiogenic therapy with bevacizumab. Different meta-analyses evaluating the predictive role of BRAF mutations show the same results in resistance to EGFR inhibitors [33].
In this panel’s experience, BRAF-V600E testing in mCRC combined with full RAS testing is needed to provide prognostic and predictive information. Sanger sequencing, RT-PCR and NGS are available across LA, but access depends on patient insurance and reimbursement. Therefore, programs sponsored by the pharmaceutical industry often provide testing to patients who are candidates for anti-EGFR therapies. LB is available in some countries; however, not all countries have granted market approval. BRAF-V600E testing is rarely performed in countries that do not have access to targeted therapies. This may be because it could be unethical to test for a condition when therapy is not accessible. However, this action may preclude patients from seeking care abroad or enrolling in clinical trials.
MSI: predictive marker of chemotherapy effectiveness in BRAF-V600E mCRC
While BRAF-V600E mutations are consistently associated with poor prognosis in mCRC, the impact of MSI-H status is more nuanced. It can vary depending on the stage of the disease and available treatment options. The combination of both factors creates a complex clinical scenario that requires careful consideration in treatment planning and prognostication [24–26].
MSI-H status in early-stage CRC (stages II and III) is generally considered a favorable prognostic factor [34]. The dMMR/MSI-H CRCs account for 15%–20% of stage II and III CRCs, representing only approximately 4% of mCRC cases; a lower frequency indicates the weakened capacity for dMMR CRCs to develop metastases [35]. When BRAF-V600E mutations and MSI-H status occur together, which happens in approximately 30% of BRAF-V600E-mutated mCRC tumors, it is generally associated with poor OS in stage III CRC [36]. However, MSI-H status in mCRC is associated with improved response to immune checkpoint blockade, potentially improving outcomes for these patients [36]. Pembrolizumab, an ICI, is indicated for treating MSI-H/dMMR mCRC patients [37]. The combination of BRAF-V600E mutation and MSI status affects the efficacy of targeted therapies. Encorafenib (BRAF inhibitor) combined with cetuximab (anti-EGFR antibody) is indicated for previously treated BRAF-V600E-mutant mCRC patients [37]. In some cases, patients with both BRAF V600E mutation and MSI-H status may show atypical response patterns to different treatment sequences [38].
The unique characteristics of BRAF V600E-mutated, MSI-H mCRC have prompted further research. The SEAMARK study evaluates the combination of pembrolizumab with encorafenib and cetuximab versus pembrolizumab alone in first-line treatment for BRAF V600E-mutant and MSI-H/dMMR mCRC [37, 39, 40]. Novel approaches include triple combinations of BRAF inhibitors, anti-EGFR antibodies and immune checkpoint inhibitors specifically for the MSI-H population [28, 38]. An assessment tree for diagnosing BRAF-V600 mCRC is depicted in Figure 2.
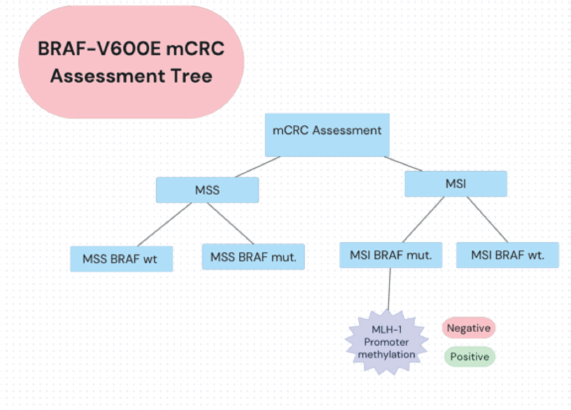
Figure 2. BRAF-V600E mCRC assessment decision tree. Oncologists in LA apply a summarised decision algorithm to detecting MSS/MSI status and mutational status among mCRC patients.
The results of these diagnostic tests are essential to guiding the therapeutic management of mCRC. Regarding evidence on the impact of immunotherapy in patients with MSI/dMMR and BRAF-V600E mutated tumors, some patients with these traits were included in the pembrolizumab and nivolumab plus or minus ipilimumab trials. In CheckMate 142, patients with histologically confirmed mCRC and MSI-H/dMMR were treated in one of three cohorts: one for first-line patients receiving nivolumab plus ipilimumab, and the other two for patients with at least two prior lines of treatment (2L), one in monotherapy with nivolumab and the other nivolumab plus ipilimumab.
The ORR in all three cohorts was encouraging, with no significant differences from those observed in the wild-type BRAF subgroup: 82% versus 62% in the first-line cohorts, 42% versus 45% in the nivolumab-alone 2L cohort and 70% versus 61% in the nivolumab-ipilimumab 2L group. Consistent with these data, there were no differences in the primary endpoint of PFS between the BRAF wild-type (HR 0.50) and BRAF-V600E (HR 0.48) groups in the Keynote 177 trial, with 77 patients carrying the BRAF-V600E mutation [47].
Therapeutic decision-making for the management of BRAF-V600E mCRC in LA
Clinical trial results and regulatory approvals impact recommended treatment decisions for managing BRAF-V600E mCRC in LA. In 2015, the phase 3 TRIBE study evaluated the chemotherapy intensification with FOLFOXIRI plus bevacizumab compared with FOLFIRI plus bevacizumab as a first-line treatment for mCRC, and 7% (28/391) of BRAF-mutated patients were identified. Results of this BRAF-mutated subgroup indicated a better median OS for the FOLFOXIRI plus bevacizumab treatment, 19 versus 10.7 months, HR 0.54 as well as a median PFS of 7.5 versus 5.5 months, and an ORR of 56 versus 42 [48].
Since those results were published, the triplet chemotherapy regimen plus bevacizumab became the standard first-line choice for this BRAF-mutated subgroup of patients. However, the TRIBE2 study compared upfront FOLFOXIRI plus bevacizumab with FOLFOX plus bevacizumab followed by FOLFIRI plus bevacizumab after disease progression did not reproduce these findings, and they reported no increased benefit from the intensified approach [48]. This might be explained by the different comparator group that was an oxaliplatin-based doublet instead of an irinotecan-based doublet as in TRIBE or by BRAF-mutated clinical heterogeneity.
A meta-analysis of five studies also showed no increased benefit from the intensified approach of FOLFOXIRI plus bevacizumab in BRAF-mutated patients. Thus, using FOLFOX plus bevacizumab can be an upfront option, leaving the triplet combination for patients needing a response, including those with the potential to convert to resectability and those with good performance status scores [48].
There are controversies concerning the use of anti-EGFR agents in BRAF-mutated patients. One meta-analysis of nine phase-3 trials and one phase-2, with 463 colorectal BRAF-mutated patients in first- and second-line treatments, showed no benefit of the anti-EGFR drug, either for median PFS (HR 0.88, p = 0.33) or median OS (HR 0.91, p 0.63), and then recommended an anti-VEGF based treatment for this group [33]. However, another meta-analysis of seven randomised trials found inadequate evidence that BRAF-mutated patients benefit differently from anti-EGFR medicines than BRAF wild-type individuals [49]. In addition, an individual patient data meta-analysis of the randomised trials from the ARCAD database found no evidence of additional efficacy of the anti-EGFRs in these patients [50].
The BEACON trial randomised 665 BRAF-V600E mCRC patients who had progressed after one or two previous regimens of encorafenib (a BRAF inhibitor), binimetinib (a MEK-inhibitor) and cetuximab (an anti-EGFR), known as the triplet arm; encorafenib; cetuximab, known as the doublet arm; or cetuximab and irinotecan-based chemotherapy, known as the control arm. Regarding the primary endpoint median OS, the findings showed 9 versus 5.4 months, favoring the triplet over the control arm; HR 0.52, p < 0.001 and 8.4 versus 5.4 months, favoring the doublet over the control arm; HR 0.60, p <0.001; ORRs were 26%, (triplet) 20%, (doublet) and 2% (control) [51]. Thus, targeted therapy in the second line can lead to survival benefits and response rate improvements.
Other options for previously treated patients came from smaller studies, including the SWOG 1406, which started with a phase-1b study combining irinotecan, cetuximab and vemurafenib [52]. Vemurafenib (a BRAF inhibitor) and the combination had a 35% ORR and 7.7 months of median PFS that led to the phase-2 study of irinotecan and cetuximab with or without vemurafenib [39]. The study randomised 106 patients and reported a median PFS benefit of 4.2 versus 2.0 months, HR of 0.50 and a p < 0.001, with an ORR of 17% versus 4%, favoring the vemurafenib arm. The crossover rate was high as 42%, and there was no significant median OS difference.
Another preliminary trial evaluated the combination of panitumumab (an anti-EGFR monoclonal antibody) and dabrafenib (a BRAF inhibitor), the combination plus trametinib (a MEK-inhibitor), and panitumumab and trametinib in 142 patients split into three arms. They found an ORR of 10%, 21% and 0%; a median PFS of 3.5, 4.2 and 2.6 months; and a median OS of 13.2, 9.1 and 8.2 months, respectively, but with poor tolerability related to skin toxicity in the triplet arm [53].
After the BEACON trial, the ANCHOR trial tested the same regimen in the first line. ANCHOR is a single-arm phase-2 trial evaluating encorafenib, binimetinib and cetuximab in BRAF-V600E-mutant mCRC first-line treatment. It showed a 50% ORR in the first 41 patients [54] and maintained a 47.8% ORR after including 95 new patients, with a median PFS of 5.8 months and a median OS of 17.2 months [55].
New chemotherapy-free, targeted treatments exist for patients with BRAF-mutated mCRC. Based on BEACON trial results, the chemotherapy-free therapy of combining encorafenib (a BRAF inhibitor) and cetuximab (an anti-EGFR antibody) was approved by the European Union (EU) in June 2020. This combination is approved for treating adult patients with BRAF-V600E mCRC who have previously received systemic therapy [40].
The ongoing phase 3 BREAKWATER trial has three arms (encorafenib, binimetinib and chemotherapy with mFOLFOX6 or the same target drugs plus chemotherapy with FOLFIRI). Preliminary results show an ORR of 68.4%, with 25.9% of participants experiencing treatment-related serious adverse events (AEs) in the mFOLFOX6 arm versus an ORR of 66.7% and 13.3% in the FOLFIRI arm [40, 56].
Currently, the recommended treatments include a cytotoxic chemotherapy combination (doublet or triplet) plus bevacizumab in the first line and a combination of a BRAF inhibitor (preferably encorafenib) with an anti-EGFR (cetuximab) with or without a MEK-inhibitor (binimetinib) in the second-line, or, if a BRAF inhibitor is unavailable, another doublet plus an anti-VEGF drug. The exception is the concomitant MSI-H status patients, who seem to benefit from ICIs as the first option [57].
In LA, socioeconomic diversity and drug availability are additional barriers to treatment. The Central America and Caribbean consensus on the management of mCRC recommended a first-line doublet (FOLFOX or FOLFIRI) or triplet (FOLFOXIRI) with an anti-VEGF antibody for patients with BRAF-mutated non-resectable cancer and for whom debulking surgery can be objective [58].
In the 2022 guidelines, the Brazilian Society of Clinical Oncology (SBOC) stated the preferred options for first-line treatment in the BRAF-V600E-mutated mCRC included doublet (mFOLFOX6 or FOLFIRI) or for young patients with aggressive disease and no comorbidities, triplet (FOLFOXIRI) plus or minus bevacizumab. The SBOC recommends including a BRAF inhibitor (encorafenib plus cetuximab plus or not binimetinib or the combination of vemurafenib plus irinotecan plus cetuximab) in second-line treatment [59].
The combination’s safety profile is similar to that of the individual medications. For anti-VEGF, the main AEs are related to thrombosis, bleeding or hypertension. The most common AEs for the chemotherapy combination are nausea, diarrhea, peripheral neuropathy (due to oxaliplatin), leukopenia, thrombocytopenia and anemia.
The BEACON trial reported that the most common AEs grade 3 or higher in the triplet arm were gastrointestinal-related ones, including diarrhea (10%), nausea (5%), vomiting (4%), abdominal pain (6%) and skin-related events (acneiform dermatitis, 2%). All grade headache (19%), musculoskeletal pain (12%), arthralgia (19%) and myalgia (13%) occurred more frequently in the doublet-therapy group (BRAF- and EGFR-inhibitors). This counterintuitive disparity in toxicity may be explained by MEK inhibition’s capacity to attenuate BRAF inhibition’s harmful effects. In this trial, the rates of AEs were similar in the triplet and the doublet arm but higher in the control arm [40].
The ANCHOR trial showed similar AEs as the BEACON trial, besides an acute kidney injury rate of grade 3 or more (5.3%). The SWOG 1406 trial showed higher grade 3 and 4 AEs in the vemurafenib arm, especially neutropenia (30 versus 7%), anemia (13 versus 0%) and nausea (19 versus 2%), along with more diarrhea and fatigue. Side effects are specific to each chemotherapy agent or a class impact for targeted treatments. Still, they are manageable, as reported in most trials [54].
Early prediction of BRAF-mutational status for informing stronger treatment decisions
The treatment of BRAF-V600E mCRC has improved over the last decade because of the parallel evolution of preclinical and clinical knowledge. Cancer immune therapy with ICIs has also shown improved results in MSI tumors. However, the true challenge in chemotherapeutics is represented by patients with BRAF-V600E dMMR tumors. Current clinical studies combining targeted medicines and checkpoint inhibitors are expected to broaden the therapy range for this subtype of CRC under the precision oncology paradigm. Overall, the recent approval of the combination of cetuximab and encorafenib represents a step forward for treating BRAF-V600E mCRC. Due to the disease’s aggressiveness, only 50% of patients reach second-line therapy. Because of this prognostic impact, it is crucial to consider early enrollment in ongoing clinical trials of all patients with BRAF-V600E mCRC.
BRAF-mutated mCRC and international standards of care
Local guideline availability for testing and treating BRAF-mutated tumors is limited in many regions, including LA. As a result, physicians often use the international standards of care for BRAF-mutated mCRC. While most countries in LA have access to some chemotherapy options for mCRC, access to novel therapeutics beyond bevacizumab is more limited. LA treatment options typically involve doublet or triplet chemotherapy regimens combined with bevacizumab and are not consistently available in all countries. The recent approval of encorafenib by the USFDA and the European Medicines Agency in April 2020 has not yet resulted in widespread access to this drug for non-clinical trial patients in LA. While encorafenib is approved in Argentina, Brazil and Chile, it is not widely available. In addition to the lack of guidelines for testing and treatment, the availability of CRC treatment is drastically limited in most LA countries, except for Brazil. Physicians often use international guidelines like those from the National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology, the American Society of Clinical Oncology (ASCO) and the ESMO.
Discussion
This panel recognises the prodigious challenge of accessing evidence-based prevention, diagnosis and treatment strategies for mCRC in LA. Molecular profiling and novel predictive biomarkers add complexity. Equanimous access to molecular diagnostics and optimum medical and surgical techniques must be promoted and ensured [60]. The region’s lack of access to effective screening programs and evidence-based surgical and clinical treatments is omnipresent. This context should be faced and prioritised as a sine qua non condition for improving patient care and reducing CRC mortality in LA over the next few decades (Table 2).
The BRAF-mutated advanced CRC is a rapidly evolving field in modern oncology. The NCCN [61], ASCO [62] and ESMO [20] have specific recommendations for BRAF-mutated CRC patients in their advanced CRC Guidelines.
To improve outcomes and prognosis for BRAF-mutated mCRC patients in LA, the panel proposes:
- Local guidelines to manage mCRC patients are necessary to contextualise cancer care to specific LA conditions. This panel favors the creation of local cancer care guidelines and for these guidelines to be adopted within LA national and local oncology societies to help clinical oncologists provide the best care to each patient V-A.
- Multidisciplinary boards to deliver better individual decisions and care to patients with mCRC. The panel recommends that each mCRC patient care plan be discussed within multidisciplinary boards of clinical and surgical specialists in gastrointestinal cancer V-A.
- Tumor tissue genotyping for all patients with mCRC. This can include BRAF mutations assayed individually or as part of a multiple genes panel. (I-A) If no tissue is available, an LB test could be used (II-B). Several validated platforms (PCR, NGS and immunohistochemistry) can perform the test. The test can be done using the primary tumor and/or the metastasis. Since there is no predictive role of BRAF testing results in localised CRC, the test should be routinely performed on relapsed/unresectable or stage IV disease I-A.
- First-line treatment for BRAF-mutated pMMR tumors should include a combination of irinotecan or oxaliplatin-based fluoropyrimidine doublets or triplets (in patients with suitable functional and performance status) with bevacizumab [63] (II-B). Patients with dMMR tumors should receive pembrolizumab as a first-line treatment [47] II-A.
- After disease progression, patients should receive second-line treatment of a BRAF inhibitor plus EGFR inhibition (encorafenib + cetuximab) (I–A) [40]. Triplet regimens with the addition of a MEK-inhibitor to BRAF- and EGFR-inhibitors are under investigation and should not be routinely recommended. Patients with BRAF-mutated tumors should not receive anti-EGFR monoclonal antibodies unless given with BRAF inhibition. Other than V600E-BRAF mutations, the best treatment is yet to be defined [64, 65] II-B.
- Citorreductive surgeries or metastasis resections should be performed according to the multidisciplinary board’s judgments, and mutation identification should not cause delaying curative procedures from oligometastatic BRAF-V600E tumors V-A.
Conclusion
In conclusion, treating patients with BRAF-V600E mCRC remains a challenge throughout LA, with opportunities for improvement in accessing diagnosis and treatment, and participating in clinical trials. While most countries in LA have access to some chemotherapy options for mCRC, access to novel therapeutics beyond bevacizumab is limited. BRAF-V600E mCRC treatment has been steadily improving because of advances in preclinical and clinical research and the emergence of cancer immune therapies with ICIs that have shown promising results in MSI tumors. However, challenges to accessing these new therapies across the region remain. Besides standard therapies for BRAF-V600E mCRC, which are limited, new chemotherapy-free targeted options have emerged in adult patients with BRAF-V600E mCRC who have received prior systemic treatment. However, due to delays in regulatory approvals in some LA countries, encorafenib is not yet widely available.
Acknowledgments
The authors thank Ms Thais Vidal, BS, for the English language editing of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
The organization and implementation of the conference were conducted by the Americas Health Foundation, a 501(c)3 nonprofit organization dedicated to improving healthcare throughout LA and was supported by an unrestricted grant from the Pfizer Foundation.
Author contributions
AKC, CB, MACG, JMOC, JCSPV, GM: Validation, formal analysis, writing – original draft.
AMJ: Visualization, project administration, writing – review and editing. MRR: Conceptualization, methodology, writing – review and editing.
GS: Resources, supervision, visualization, writing – review and editing.
Disclosure of results at a meeting
The results of this paper were presented as a poster at the 25th World Congress on Gastrointestinal Cancer on 28 June 2023.
References
1. Sanz-Garcia E, Argiles G, and Elez E, et al (2017) BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives Ann Oncol 28(11) 2648–2657 https://doi.org/10.1093/annonc/mdx401 PMID: 29045527
2. Kayhanian H, Goode E, and Sclafani F, et al (2018) Treatment and survival outcome of BRAF-mutated metastatic colorectal cancer: a retrospective matched case-control study Clin Colorectal Cancer 17(1) e69–e76 https://doi.org/10.1016/j.clcc.2017.10.006
3. Schmiegel W, Reinacher-Schick A, and Arnold D, et al (2013) Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: a randomized phase II study of the AIO colorectal study group Ann Oncol 24(6) 1580–1587 https://doi.org/10.1093/annonc/mdt028 PMID: 23463625
4. Clarke CN and Kopetz ES (2015) BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies J Gastrointest Oncol 6(6) 660–667 PMID: 26697199 PMCID: 4671844
5. Sinicrope FA, Shi Q, and Smyrk TC, et al (2015) Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes Gastroenterology 148(1) 88–99 https://doi.org/10.1053/j.gastro.2014.09.041
6. Jones JC, Renfro LA, and Al-Shamsi HO, et al (2017) (Non-V600) BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer J Clin Oncol 35(23) 2624–2630 https://doi.org/10.1200/JCO.2016.71.4394 PMID: 28486044 PMCID: 5549454
7. Safaee Ardekani G, Jafarnejad SM, and Tan L, et al (2012) The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis PLoS One 7(10) e47054 https://doi.org/10.1371/journal.pone.0047054 PMID: 23056577 PMCID: 3467229
8. Hernandez-Sandoval JA, Gutierrez-Angulo M, and Magana-Torres MT, et al (2020) Prevalence of the BRAF p.v600e variant in patients with colorectal cancer from Mexico and its estimated frequency in Latin American and Caribbean populations J Investig Med 68(5) 985–991 https://doi.org/10.1136/jim-2020-001301 PMID: 32184228 PMCID: 7306871
9. Luévano-González A, Guzmán AQ, and Ancer Rodríguez J, et al (2011) Analysis of DNA mismatch repair proteins expression and BRAF V600E mutation in a subset of early- and late-onset colorectal carcinoma patients in Mexico Arch Med Res 42(6) 457–462 https://doi.org/10.1016/j.arcmed.2011.09.008 PMID: 21945875
10. González-Colunga KJ, Lino-Silva LS, and Salcedo-Hernández RA, et al (2020) BRAF V600E expression by immunohistochemistry in colon cancer and clinico-pathologic features associated with BRAF-mutated colonic cancers in Mexican patients J Gastrointest Cancer 51(1) 35–40 https://doi.org/10.1007/s12029-018-00191-9
11. Perazzo F, Denninghoff V, and Pasccon G, et al (2009) Preliminary report of the mutation status of KRAS and BRAF-V600E in an Argentinean population of primary colorectal tumors J Clin Oncol 27(15_suppl) e22183 https://doi.org/10.1200/jco.2009.27.15_suppl.e22183
12. Rasuck CG, Leite SM, and Komatsuzaki F, et al (2012) Association between methylation in mismatch repair genes, V600E BRAF mutation and microsatellite instability in colorectal cancer patients Mol Biol Rep 39(3) 2553–2560 https://doi.org/10.1007/s11033-011-1007-8
13. Yamane LS, Scapulatempo-Neto C, and Alvarenga L, et al (2014) KRAS and BRAF mutations and MSI status in precursor lesions of colorectal cancer detected by colonoscopy Oncol Rep 32(4) 1419–1426 https://doi.org/10.3892/or.2014.3338 PMID: 25050586
14. dos Santos W, Sobanski T, and de Carvalho AC, et al (2019) Mutation profiling of cancer drivers in Brazilian colorectal cancer Sci Rep 9(1) 13687 https://doi.org/10.1038/s41598-019-49611-1 PMID: 31548566 PMCID: 6757044
15. Hurtado C, Wielandt AM, and Zárate AJ, et al (2015) Análisis molecular del cáncer de colon esporádico Rev Méd Chile 143 310–319 https://doi.org/10.4067/S0034-98872015000300005
16. Roa I, Game A, and Bizama C, et al (2014) Mutación del gen BRAF en pacientes con cánceres de colon y recto con KRAS no mutado Rev Méd Chile 142 55–60 https://doi.org/10.4067/S0034-98872014000100009
17. Egoavil CM, Montenegro P, and Soto JL, et al (2011) Clinically important molecular features of Peruvian colorectal tumours: high prevalence of DNA mismatch repair deficiency and low incidence of KRAS mutations Pathology 43(3) 228–233 https://doi.org/10.1097/PAT.0b013e3283437613 PMID: 21436632
18. Montenegro PC, Egoavil C, and Casanova LA, et al (2010) Molecular features of colorectal cancer in Peruvian patients J Clin Oncol 28(15_suppl) e14043 https://doi.org/10.1200/jco.2010.28.15_suppl.e14043
19. Wielandt AM, Villarroel C, and Hurtado C, et al (2017) Caracterización de pacientes con cáncer colorrectal esporádico basado en la nueva subclasificación molecular de consenso Rev Med Chil 145(4) 419–430 https://doi.org/10.4067/S0034-98872017000400001 PMID: 28748988
20. Cervantes A, Adam R, and Rosello S, et al (2022) Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up Ann Oncol PMID: 36307056
21. Guttlein L, Luca MR, and Esteso F, et al (2022) Liquid biopsy for KRAS, NRAS and BRAF mutation testing in advanced colorectal cancer patients: the Argentinean experience Future Oncol 18(29) 3277–3287 https://doi.org/10.2217/fon-2022-0329 PMID: 36004810
22. Clinica AAdO (2021) Recomendaciones actuales para el tratamiento oncologico (Cancer colorectal metastásico)
23. Peixoto RDA, Riechelmann RP, and Prolla G, et al (2019) Treatment choices in metastatic colorectal cancer according to sidedness and RAS/BRAF status: a national survey by the Brazilian Gastrointestinal Tumors Group (GTG) Braz J Oncol https://doi.org/10.5935/2526-8732.20190021
24. Resumen G (2018) Guía de Práctica Clínica: Manejo Multidisciplinario del Cáncer de Colon (MMCC) (Peru: AUNA)
25. Torrecillas-Torres L, Cervantes-Sánchez MG, and Adame-González I, et al (2022) Recomendaciones para diagnóstico y tratamiento del cáncer de colon y recto en México Gac Mexi Oncol 18(4)
26. Cátedra de Oncología Clínica (2020) Pautas de oncologia médica para el diagnostico, tratamiento sistemico y seguimiento
27. De Roock W, Claes B, and Bernasconi D, et al (2010) Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis Lancet Oncol 11(8) 753–762 https://doi.org/10.1016/S1470-2045(10)70130-3 PMID: 20619739
28. Venderbosch S, Nagtegaal ID, and Maughan TS, et al (2014) Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies Clin Cancer Res 20(20) 5322–5330 https://doi.org/10.1158/1078-0432.CCR-14-0332 PMID: 25139339 PMCID: 4201568
29. Roth AD, Tejpar S, and Delorenzi M, et al (2010) Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial J Clin Oncol 28(3) 466–474 https://doi.org/10.1200/JCO.2009.23.3452
30. Taieb J, Shi Q, and Pederson L, et al (2019) Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies Ann Oncol 30(9) 1466–1471 https://doi.org/10.1093/annonc/mdz208 PMID: 31268130 PMCID: 7360150
31. Lochhead P, Kuchiba A, and Imamura Y, et al (2013) Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication J Natl Cancer Inst 105(15) 1151–1156 https://doi.org/10.1093/jnci/djt173 PMID: 23878352 PMCID: 3735463
32. Lee S, Cho NY, and Choi M, et al (2008) Clinicopathological features of CpG island methylator phenotype-positive colorectal cancer and its adverse prognosis in relation to KRAS/BRAF mutation Pathol Int 58(2) 104–113 https://doi.org/10.1111/j.1440-1827.2007.02197.x PMID: 18199160
33. Pietrantonio F, Petrelli F, and Coinu A, et al (2015) Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis Eur J Cancer 51(5) 587–594 https://doi.org/10.1016/j.ejca.2015.01.054 PMID: 25673558
34. Guastadisegni C, Colafranceschi M, and Ottini L, et al (2010) Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data Eur J Cancer 46(15) 2788–2798 https://doi.org/10.1016/j.ejca.2010.05.009 PMID: 20627535
35. Baretti M and Le DT (2018) DNA mismatch repair in cancer Pharmacol Ther 189 45–62 https://doi.org/10.1016/j.pharmthera.2018.04.004 PMID: 29669262
36. Park R, da Silva LL, and Lee S, et al (2021) Impact of BRAF mutations on prognosis and immunotherapy response in microsatellite instability/mismatch repair deficient metastatic colorectal cancer: a systematic review and meta-analysis J Clin Oncol 39 3557 https://doi.org/10.1200/JCO.2021.39.15_suppl.3557
37. Kopetz SE, Bekaii-Saab TS, and Yoshino T, et al (2022) SEAMARK: randomized phase 2 study of pembrolizumab + encorafenib + cetuximab versus pembrolizumab alone for first-line treatment of BRAF V600E-mutant and microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) metastatic colorectal cancer (CRC) J Clin Oncol 40 TPS3634 https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS3634
38. Gallois C, Taieb J, and Sabouret A, et al (2022) Upfront progression under pembrolizumab followed by a complete response after encorafenib and cetuximab treatment in BRAF V600E-mutated and microsatellite unstable metastatic colorectal cancer patient: a case report Genes Chromosomes Cancer 61(2) 114–118 https://doi.org/10.1002/gcc.23012
39. Kopetz S, Guthrie KA, and Morris VK, et al (2021) Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG s1406) J Clin Oncol 39(4) 285–294 https://doi.org/10.1200/JCO.20.01994 PMCID: 8462593
40. Kopetz S, Grothey A, and Yaeger R, et al (2019) Encorafenib, binimetinib, and cetuximab in BRAF v600e-mutated colorectal cancer N Engl J Med 381(17) 1632–1643 https://doi.org/10.1056/NEJMoa1908075 PMID: 31566309
41. Dudley JC, Lin MT, and Le DT, et al (2016) Microsatellite instability as a biomarker for PD-1 blockade Clin Cancer Res 22(4) 813–820 https://doi.org/10.1158/1078-0432.CCR-15-1678 PMID: 26880610
42. Samowitz WS, Sweeney C, and Herrick J, et al (2005) Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers Cancer Res 65(14) 6063–6069 https://doi.org/10.1158/0008-5472.CAN-05-0404 PMID: 16024606
43. Buttner R and Friedrichs N (2019) Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany Pathologe 40(6) 584–591 PMID: 31372733
44. Tan E, Whiting J, and Xie H, et al (2022) BRAF mutations are associated with poor survival outcomes in advanced-stage mismatch repair-deficient/microsatellite high colorectal cancer Oncologist 27(3) 191–197 https://doi.org/10.1093/oncolo/oyab055 PMID: 35274712 PMCID: 8914499
45. Rau TT, Dawson H, and Hartmann A, et al (2017) Hereditary colorectal cancer: an update on genetics and entities in terms of differential diagnosis Pathologe 38(3) 156–163 https://doi.org/10.1007/s00292-017-0294-9 PMID: 28474162
46. Bucksch K, Zachariae S, and Aretz S, et al (2020) Cancer risks in Lynch syndrome, Lynch-like syndrome, and familial colorectal cancer type X: a prospective cohort study BMC Cancer 20(1) 460 https://doi.org/10.1186/s12885-020-06926-x PMID: 32448342 PMCID: 7245918
47. Andre T, Shiu KK, and Kim TW, et al (2020) Pembrolizumab in microsatellite-instability-high advanced colorectal cancer N Engl J Med 383(23) 2207–2218 https://doi.org/10.1056/NEJMoa2017699 PMID: 33264544
48. Cremolini C, Antoniotti C, and Rossini D, et al (2020) Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial Lancet Oncol 21(4) 497–507 https://doi.org/10.1016/S1470-2045(19)30862-9 PMID: 32164906
49. Rowland A, Dias MM, and Wiese MD, et al (2015) Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer Br J Cancer 112(12) 1888–1894 https://doi.org/10.1038/bjc.2015.173 PMID: 25989278 PMCID: 4580381
50. Karapetis CS, Liu H, and Sorich M, et al (2020) Treatment effects (TEs) of EGFR monoclonal antibodies (mAbs) in metastatic colorectal cancer (mCRC) patients (pts) with KRAS, NRAS, and BRAF mutation (MT) status: individual patient data (IPD) meta-analysis of randomized trials from the ARCAD database Am Soc Clin Oncol 38(15_suppl) 4090 https://doi.org/10.1200/JCO.2020.38.15_suppl.4090
51. Kopetz S, Grothey A, and Yaeger R, et al (2021) BREAKWATER: randomized phase 3 study of encorafenib (enco) + cetuximab (cetux) ± chemotherapy for first-line (1L) treatment (tx) of BRAF V600E-mutant (BRAFV600E) metastatic colorectal cancer (mCRC) J Clin Oncol 39(15_suppl) TPS3619 https://doi.org/10.1200/JCO.2021.39.15_suppl.TPS3619
52. Hong DS, Morris VK, and El Osta B, et al (2016) Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with brafv600e mutation Cancer Discov 6(12) 1352–1365 https://doi.org/10.1158/2159-8290.CD-16-0050 PMID: 27729313 PMCID: 5562357
53. Corcoran RB, Andre T, and Atreya CE, et al (2018) Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer Cancer Discov 8(4) 428–443 https://doi.org/10.1158/2159-8290.CD-17-1226 PMID: 29431699 PMCID: 5882509
54. Grothey A, Tabernero J, and Taieb J, et al (2020) LBA-5 ANCHOR CRC: a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer Ann Oncol 31 S242–S243 https://doi.org/10.1016/j.annonc.2020.04.080
55. Van Cutsem E, Taieb J, and Yaeger R, et al (2021) O-10 ANCHOR CRC: results from a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E–mutant metastatic colorectal cancer Ann Oncol 32(Suppl 3) https://doi.org/10.1016/j.annonc.2021.05.014
56. Tabernero J, Yoshino T, and Kim TW, et al (2022) LBA26 BREAKWATER safety lead-in (SLI): encorafenib (E) + cetuximab (C) + chemotherapy (chemo) for BRAFV600E metastatic colorectal cancer (mCRC) Ann Oncol 33 S1392–S1393 https://doi.org/10.1016/j.annonc.2022.08.022
57. Mauri G, Bonazzina E, and Amatu A, et al (2021) The evolutionary landscape of treatment for BRAF(V600E) mutant metastatic colorectal cancer Cancers (Basel) 13(1) 137 https://doi.org/10.3390/cancers13010137 PMID: 33406649 PMCID: 7795863
58. Lopez RI, Castro JL, and Cedeno H, et al (2018) Consensus on management of metastatic colorectal cancer in Central America and the Caribbean: San Jose, Costa Rica, August 2016 ESMO Open 3(3) e000315 https://doi.org/10.1136/esmoopen-2017-000315
59. D’Alpino R, dos Anjos AA, and Siquiera MB, et al (2022) Diretrizes de tratamentos oncológicos recomendados pela Sociedade Brasileira de Oncologia Clinica (Sociedade Brasileira de Oncologia Clínica)
60. Pan American Health Organization (2016) Colorectal Cancer Screening in the Americas: Situation and challenges (Washington: Pan American Health Organization)
61. Benson A, Venook A, and Al Hawary M, et al (2022) Colon cancer J Natl Compr Cancer Netw 2(12) 198
62. Morris VK, Kennedy EB, and Baxter NN, et al (2022) Treatment of metastatic colorectal cancer: ASCO guideline J Clin Oncol
63. Loupakis F, Cremolini C, and Masi G, et al (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer N Engl J Med 371(17) 1609–1618 https://doi.org/10.1056/NEJMoa1403108 PMID: 25337750
64. Yaeger R, Kotani D, and Mondaca S, et al (2019) Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer Clin Cancer Res 25(23) 7089–7097 https://doi.org/10.1158/1078-0432.CCR-19-2004 PMID: 31515458 PMCID: 6891165
65. Sartore-Bianchi A, Pietrantonio F, and Lonardi S, et al (2021) Phase II study of anti-EGFR rechallenge therapy with panitumumab driven by circulating tumor DNA molecular selection in metastatic colorectal cancer: the CHRONOS trial J Clin Oncol 39(15_suppl) 3506 https://doi.org/10.1200/JCO.2021.39.15_suppl.3506






