The significance of the site of origin in primary peritoneal carcinosarcoma: case report and literature review
Anupama Rajanbabu1, Sheikh Zahoor Ahmad2, Vijaykumar D K3, Pavithran K4, and Santhosh Kuriakose5
1 Department of Surgical and Gynecologic Oncology, Amrita Institute of Medical Sciences, Amrita Vishwavidyapeetham, India
2 Department of Surgical Oncology, Florence hospital, Srinagar, Kashmir, India
3 Department of Surgical and Gynecologic Oncology, Amrita Institute of Medical Sciences, Amrita Vishwavidyapeetham, India
4 Department of Medical Oncology, Amrita Institute of Medical Sciences, Amrita Vishwavidyapeetham, India
5 Department of Gynecology, Govt. Medical College, Calicut, India
Correspondence to: Anupama Rajanbabu. Email: anupamashyam@gmail.com
Abstract
Primary peritoneal carcinomas are rare, highly aggressive malignant neoplasms containing both sarcomatous and carcinomatous elements. Surgical debulking is the mainstay of treatment for primary peritoneal carcinomas. Systemic chemotherapy is advised in all cases because of the early spreading of these tumours. We report on a case of primary peritoneal carcinosarcoma occurring in a 22-year-old woman.
Keywords: primary peritoneal carcinosarcoma, malignant mixed Mullerian tumour, extrauterine carcinosarcoma
Carcinosarcomas are highly aggressive biphasic neoplasms composed of carcinomatous and sarcomatous elements. Mostly occurring in the female genital tract in elderly postmenopausal women, they have also been described in head and neck, gastrointestinal tract, biliary tract and peritoneum [1]. Primary peritoneal carcinosarcomas are extremely rare with the majority of the tumours occurring in the pelvic peritoneum, followed by decreasing frequency on the serosal surface of the colon, retroperitoneum, anterolateral abdominal peritoneum, and omentum [2]. They have poor outcomes despite being managed with upfront surgery and chemotherapy [3]. After the first case described by Ober and Black [4] in 1955, only 35 cases have been reported in the English literature. So far, all the reported cases are in females over the age of 40. We report a case of primary peritoneal carcinosarcoma occurring in a 22-year-old woman.
Case history
A 22-year-old unmarried female presented with a two month history of non-colicky lower abdominal pain. Clinically, there was a 10 × 12 cm mobile mass arising from the pelvis reaching up to the umbilicus. Contrast enhanced computerised Tomography (CECT) abdomen revealed an 11 × 11 × 9 cm heterogeneously enhancing predominantly cystic mass in the lower abdomen superior to the bladder and uterus extending upwards to aortic bifurcation. It was abutting the anterior surface of the left psoas muscle and left external iliac artery, and anteriorly reaching up to the parietal wall. The ovaries, uterus, and rest of the viscera were normal. Serum tumour markers (CA-125, AFP, and ß-HCG) were within normal limits. The patient underwent an exploratory laparotomy. Intra-operatively minimal hemorrhagic ascites were noted. A 12 × 10 cm heterogeneous solid cystic mass was noted in the sigmoid mesentery adherent to the psoas fascia posteriorly. Both ovaries and uterus were seen to be normal. The mass was excised completely with a segment of sigmoid colon.
Gross pathology showed an intact 13 × 9 × 10 cm mass with a segment of sigmoid colon. Cut section showed grey white fleshy solid areas, interspersed areas of haemorrhage, along with multiple cystic spaces filled with thick fluid. Microscopic examination showed that the cystic areas were lined by tall columnar cells arranged in papillary and cribriform pattern and with pale oeosinophilic cytoplasm and pleomorphic nuclei. Solid areas showed sheets of spindly cells with moderate cytoplasm and elongated pleomorphic nuclei. There were five to six mitosis/10 HPF. Neoplasm was adherent to the serosa of the sigmoid colon. Adjacent colonic mucosa was normal. The epithelial cells were positive for cytokeratin (CK) and focally for carcinoembryonic antigen (CEA) and the spindly cells were positive for vimentin (Figure 1).
Post operatively she was given six cycles of chemotherapy with ifosfamaide and cisplatin. CT scan taken after completion of chemotherapy showed a recurrent lesion within the abdomen measuring 6 × 5.8 × 4.3 cm size. In view of the progressive disease, carboplatin, and paclitaxel combination chemotherapy was started. CT scan taken after two cycles showed that the disease was progressing on treatment and hence, a cycle of Adriamycin with cyclophosphomide was given. There was no clinical response with the third line chemotherapy also and the patient expired 9 months after starting the treatment.
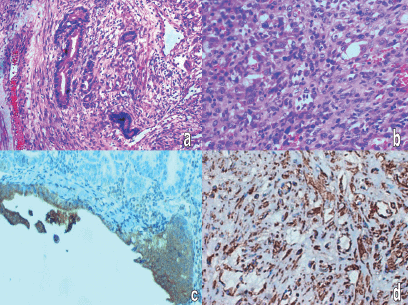
Figure 1: Histopathology: (a) adenocarcinomatous area -H&E-400 X, (b) sarcomatous area with giant cells and atypical mitosis H&E-400 X, (c) cytokeratin positivity in carcinomatous area, (d) vimentin positivity in sarcomatous area.
Discussion
Carcinosarcomas or malignant mixed Mullerian tumours are rare, highly aggressive biphasic neoplasms comprised of carcinomatous and sarcomatous components, which accounts for one to three per cent of all uterine malignancies [5] and even more rarely are described to occur in head and neck, gastrointestinal tract, biliary tract and peritoneum [1]. The carcinosarcomas at extragenital sites have been postulated to arise from pre-existing foci of endometriosis, Mullerian duct remnants, or secondary Mullerian system; all of which are derivatives of coelomic epithelium [6]. The histopathological features of this tumour are identical to uterine carcinosarcomas. Uterine carcinomas are now classified as high-grade carcinomas since in vitro studies, immunohistochemistry, and molecular studies have shown that they are derived from monoclonal cancer cells which exhibit sarcomatous metaplasia [7] and the behaviour of these tumours is determined by the epithelial component.
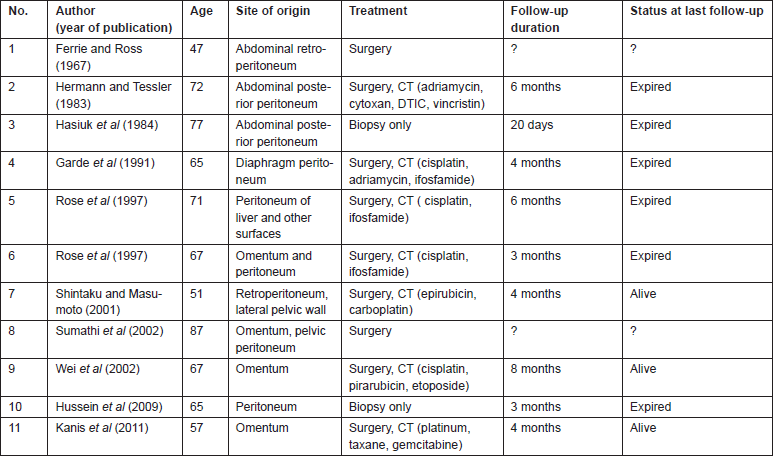
Literature search revealed 35 cases of primary peritoneal carcinosarcomas [8, 12–15]. The site of origin varied from pelvic and cul-de-sac peritoneum, uterine serosa, colonic or rectal serosa to abdominal wall peritoneum or retroperitoneum and omentum. We classified the patients into three groups based on the site of origin of tumour; Group 1 arising from pelvic peritoneum/uterine serosa, Group 2 arising from the colonic/rectal peritoneum, Group 3 arising from other peritoneal surfaces. Fourteen patients had tumour arising from the pelvic peritoneum/uterine serosa (Table 1), 11 patients had tumour arising from colonic/rectal peritoneum (Table 2) and 11 patients had tumour arising from omentum, retroperitoneum or abdominal wall peritoneum (Table 3).
Table 1: Primary peritoneal carcinosarcomas arising from the pelvic peritoneum/uterine serosa.
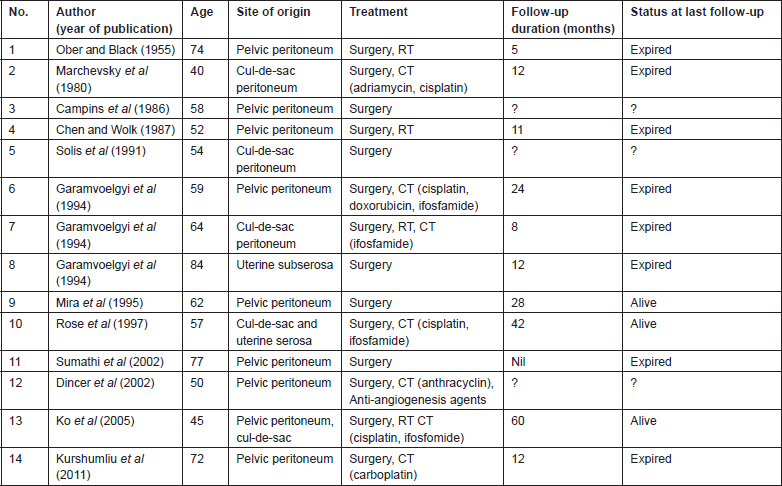
Table 2. Primary peritoneal carcinosarcomas arising from the colonic/rectal peritoneum.
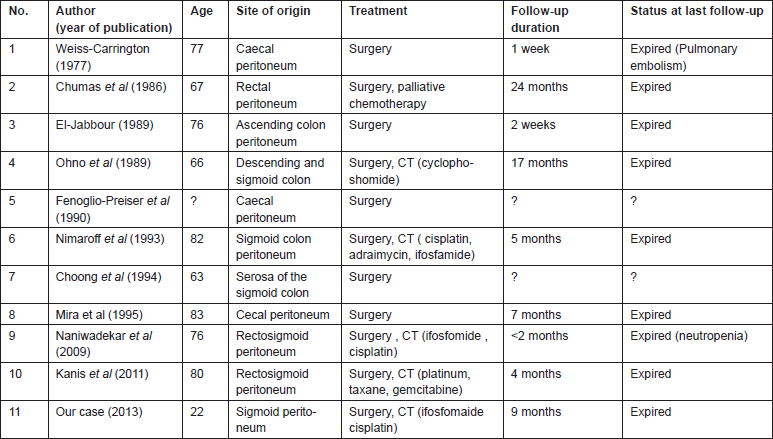
Table 3. Primary peritoneal carcinosarcomas arising from other peritoneal surfaces.
Owing to the rarity of primary peritoneal carcinosarcomas, limited data regarding the management exists. The mainstay of treatment is surgical debulking, but most cases of carcinosarcomas have wide spread metastasis at the time of presentation, making optimal tumour debulking difficult [8]. Systemic chemotherapy is advised in all cases irrespective of stage because of early tumour spread. Platinum in combination with ifosfamide were the preferred agents [9]. However, if the tumour behaviour is determined by the epithelial component, data regarding chemotherapy can be extrapolated from the treatment for ovarian cancers. Platinum and taxane combination chemotherapy has given more than 2 years median survival in patients with ovarian carcinosarcoma [10, 11]. It seems logical that these should be used as first line agents for patients with primary peritoneal carcinosarcomas also. There is not enough data to support the role of radiotherapy in extragenital carcinosarcoma [12].
Literature review shows that all patients were managed by surgery followed by chemotherapy when possible (Tables 1–3). Overall survival of all the three groups was compared. Patients belonging to Group 1, who had disease arising mostly from pelvic peritoneum and cul-de-sac, were found to have an average survival of 21.5 months. Group 2 patients with tumour arising from colonic/rectal peritoneum had an average survival of 7.6 months, and the third group had an average survival of only 4.3 months. Log rank analysis (Mantel–Cox) showed that survival time of Group 2 and Group 3 are significantly different from Group 1 (p = 0.016 and p = 0.040). There is no significant difference in survival between Group 2 and Group 3 (p = 0.696). This indicates that a patient with primary peritoneal carcinosarcoma with tumour originating from pelvic peritoneum or uterine serosa is having better survival than a patient with tumour originating from other peritoneal surface. The Kaplan–Meir survival graph is given in Figure 2.
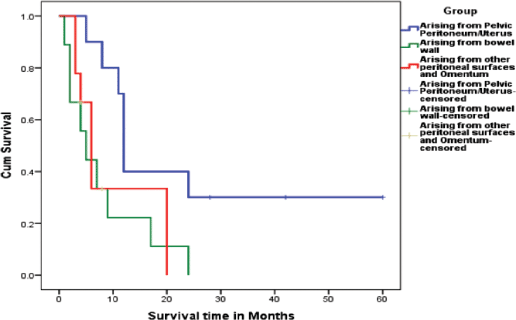
Figure 2: Kaplan Meir survival graph showing the difference in survival between the three groups.
Conclusion
Primary peritoneal carcinosarcomas are rare tumours associated with a poor prognosis. Tumours arising from the bowel serosa, abdominal wall, omentum or retroperitoneum seem to carry a poorer prognosis than those arising from the pelvic peritoneum. Since these tumours are similar to uterine carcinosarcomas where recent evidence has shown that the tumour behaviour is dictated by the epithelial component, chemotherapy with platinum taxane combination may work better as first line therapy.
References
1. Ambrosini-Spaltro A, Vaira V, Braidotti P, Rovati MP, Ferrero S, Bosari S. Carcinosarcoma of the colon: report of a case with morphological, ultrastructural and molecular analysis. BMC Cancer 6 185. DOI: 10.1186/1471-2407-6-185 PMID: 16836749 PMCID: 1570146
2. Shen DH, Khoo US, Xue WC, Ngan HY, Wang JL, Liu VW, et al. Primary peritoneal malignant mixed Mullerian tumors. A clinicopathologic, immunohistochemical, and genetic study Cancer 91:1052–60 DOI: 10.1002/1097-0142 PMID: 11251959
3. Mikami M, Kuwabara Y, Tanaka K, Komiyama S, Ishikawa M, Hirose T. Malignant mixed mullerian tumor of primary mesenteric origin. Int J Gynecol Cancer 15 1249–53 DOI: 10.1111/j.1525-1438.2005.00192.x PMID: 16343225
4. Ober WB and Black MB (1955) Neoplasms of the subcoelomic mesenchyme: report of two cases Arch Pathol Lab Med 59 698–705
5. Cantrell LA, Havrilesky L, Moore DT et al (2012) A multiinstitutional cohort study of adjuvant therapy in stage I-II uterine carcinosarcoma Gynecol Oncol 127 22–26 DOI: 10.1016/j.ygyno.2012.06.020 PMID: 22727985
6. Ibanez-Manlapaz IG, Coy DM, Vincent V 3rd, Ule UJ. Malignant Mixed Mullerian Tumor of the Extra-genital Coelomic Epithelium: Report of two cases Pathol Oncol Res 3 130–134. DOI: 10.1007/BF02907808 PMID: 11173640
7. McCluggage WG (2002) Uterine carcinosarcomas (malignant mixed mullerian tumors) are metaplastic carcinomas Int J Gynecol Cancer 12 687–90 DOI: 10.1046/j.1525-1438.2002.01151.x PMID: 12445244
8. Hussein MR, Hussein SR, Abd-Elwahed AR. Primary peritoneal malignant mixed mesodermal (Mullerian) tumor Tumori 95:525–31 PMID: 19856669
9. Gonzalez Bosquet J, Terstriep SA, Cliby WA, Brown-Jones M, Kaur JS, Podratz KC, Keeney GL. The impact of multi-modal therapy on survival for uterine carcinosarcomas Gynecol Oncol 116 419–23 DOI: 10.1016/j.ygyno.2009.10.053
10. Duska LR, Garrett A, Eltabbakh GH, Oliva E, Penson R, Fuller AF. Paclitaxel and platinum chemotherapy for malignant mixed mullerian tumors of the ovary Gynecol Oncol 85 459–63 DOI: 10.1006/gyno.2002.6645 PMID: 12051874
11. Leiser AL, Chi DS, Ishill NM, Tew WP. Carcinosarcoma of the ovary treated with platinum and taxane: the memorial Sloan-Kettering Cancer Center experience Gynecol Oncol 105 657–61 DOI: 10.1016/j.ygyno.2007.01.037 PMID: 17395252
12. Ko ML, Jeng CJ, Huang SH, Shen J, Tzeng CR, Chen SC. Primary peritoneal carcinosarcoma (malignant mixed Mullerian tumor): Report of a case with five-year disease free survival after surgery and chemoradiation and a review of literature Acta Oncol 44 756–60 DOI: 10.1080/02841860500252016 PMID: 16227168
13. Naniwadekar MR, Desai SR, Ranade RG, et al. Extra genital heterologous malignant mixed mullerian tumor of primary peritoneal origin Indian J Pathol Microbiol 52(1) 88–90. DOI: 10.4103/0377-4929.44976 PMID: 19136793
14. Kurshumliu F, Rung-Hansen H, Skovlund VR, Gashi-Luci L, Horn T. Primary malignant mixed müllerian tumor of the peritoneum a case report with review of the literature World J Surg Oncol 9 17 DOI: 10.1186/1477-7819-9-17 PMID: 21294883 PMCID: 3039619
15. Kanis M, Kesterson JP, Shroff S, Lele S, Mhawech-Faucegial. Malignant mixed müllerian tumor of primary peritoneal origin Ann Diagn Pathol 15(4) 273–7 DOI: 10.1016/j.anndiagpath.2010.03.004






