Uterine sarcoma: A 10-year retrospective analysis of clinicopathological and prognostic factors of surgically treated cases
Japhia David, Ruchi Arora, Abhilash Vasanth, Kaustubh Burde, Bijal Patel and Chetana Parekh
Department of Gynaecological Oncology, The Gujarat Cancer and Research Institute, Ahmedabad, Gujarat, India
Abstract
Background: This study aims to analyse the clinicopathological features of uterine sarcoma (US) and its impact on the survival outcomes.
Methods: This is a retrospective observational study including 85 patients of histologically proven US, surgically treated during the period of January 2010 to December 2019. The patients were staged according to the FIGO staging system. Surgery was the mainstay of management with adjuvant treatment administered based on the stage and histopathological subtype. Descriptive analysis of the sociodemographic and clinicopathological features was done and survival analysis was done using Kaplan-Meier method.
Results: The mean age of presentation in our study was 50.8 years for uterine leiomyosarcoma (ULMS), 45.5 years for low grade endometrial stromal sarcoma (LGESS), 48.1 years for high grade endometrial stromal sarcoma (HGESS) and 49.6 years in the OTHERS group. The majority were diagnosed in stage I (ULMS = 37.6%, LGESS = 16.5%, HGESS = 4.7%; OTHERS = 4.7%). The overall recurrence rate was 56.5%. 5-year overall survival (OS) and disease-free survival (DFS) rate of adenosarcoma (AS) was 100%, respectively. LGESS showed a 5-year OS and DFS rate of 86.3% and 60.5%, respectively. The 5-year OS and DFS rate of ULMS was calculated to be 77.7% and 39.9%, respectively. The 3-year OS and DFS of HGESS has been calculated to be 58.3% and 22.2%, respectively. Significance of serum lactate dehydrogenase value, stage of the tumour and the nature of adjuvant treatment were demonstrated for recurrence.
Conclusion: US tend to recur. However, AS and LGESS show better survival outcomes as per our study. A proper workup with intervention including appropriate adjuvant treatment would help to mitigate the severity of the disease.
Keywords: uterine sarcoma, overall survival, disease-free survival, recurrence, prognosis
Correspondence to: Ruchi Arora
Email: ruchi.arora@gcriindia.org
Published: 09/01/2026
Received: 05/06/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Uterine sarcomas (US) constitute a rare group of gynaecological malignancies arising from the uterine mesenchymal cells [1]. They account for about 3% of all uterine malignancies and 1% of all female tract malignancies [1, 2]. Uterine Leiomyosarcoma (ULMS) is identified as the most frequent pathological subtype as per previous studies with an incidence of 0.86 per 1 lakh women [3, 4]. Other often-identified subtypes include, endometrial stromal sarcoma (ESS) further subclassified as high grade ESS (HGESS) and low grade ESS (LGESS) and undifferentiated US (UUS). A few uncommon subtypes include adenosarcoma (AS), perivascular epithelioid tumours and uterine tumours resembling ovarian sex cord stromal tumours. USs are considered to be aggressive tumours with poor prognoses [5]. However, the prognosis depends largely upon the histopathological type [6]. Surgery remains as the mainstay of management depending on the stage [7]. This study aims to analyse the clinicopathological features of US and its impact on the survival of patients who had undergone surgery.
Material and methods
This is a retrospective single institutional observational study done at The Gujarat Cancer and Research Institute, Ahmedabad, India including the period of January 2010 to December 2019. The data of 85 patients surgically treated during the study period were recruited for the study. Ethical clearance was obtained from the institutional ethics committee of our institute vide number IRC/2025/P-58.
This study included all the patients with histopathologically proven diagnoses of US either primarily diagnosed and treated at our institute or treated outside but had their histopathological slide reviewed at out institute and continued strict follow-up at our institute. Follow up included physical visits and telephonic conversations regarding the survival status. The patients with improper data and improper follow-up were excluded from the study.
The patients were staged according to the FIGO staging (2014). Surgery was the mainstay of management. Surgery included Total Abdominal Hysterectomy (TH) with bilateral or unilateral salpingo-oophorectomy (USO/BSO). Omentectomy and lymph node dissection (LND) were done in a few earlier cases. Adjuvant treatment in the form of chemotherapy, radiotherapy or hormonal therapy was administered depending on the stage and histopathological subtype. The preferred chemotherapeutic drug regimen was Docetaxel and Gemcitabine. Radiotherapy was rendered in ways of External Beam Radiotherapy (EBRT) of 50 gray in 25 fractions or Brachytherapy. Letrozole, an aromatase inhibitor at a dose of 2.5 mg was the preferred hormonal drug. The appropriate adjuvant treatment was decided as per multidisciplinary tumour board discussions.
The data compilation included basic socio-demographic data and clinicopathological features such as tumour size and site, preoperative radiological findings, tumour markers such as serum CA-125, Lactate dehydrogenase (LDH), Carcinoembryonic antigen (CEA), CA 19-9 and hormone statuses such as Estrogen receptor (ER) and Progesterone receptor (PR) status on immunohistochemistry. The data regarding the modality of surgery, adjuvant treatment and details of recurrence also were gathered for analysis. The data were categorised according to the pathological subclasses as, ULMS, HGESS and LGESS. The variants such as AS, UUS, alveolar rhabdomyosarcoma (ARMS) and embryonal rhabdomyosarcoma (ERMS) were collectively categorised under a group named ‘OTHERS’ due to paucity of cases. The data were retrieved from the computerised data information system and case files from medical records department of our institute.
Statistical analysis was done using SPSS software version 23.0. Descriptive method using measures of dispersion was used to analyse the socio-demographic data. Survival outcome analysis was done using the Kaplan–Meier method. Univariate analysis was used to correlate the prognostic factors associated with overall survival (OS) and disease-free survival (DFS). A p-value of <0.05 was considered significant in the study.
Results
Out of the 85 cases taken up for the study, there were 44 cases of ULMS, 25 cases of LGESS, 9 cases of HGESS and 7 cases under the OTHERS category. The OTHERS category included one case of AS with sarcomatous overgrowth (SO), two cases of AS without SO, two cases of UUS, one case of ERMS and one case of ARMS.
Clinical characteristics are described in Table 1. The mean age of presentation in our study was 50.8 years for ULMS, 45.5 years for LGESS, 48.1 years for HGESS and 49.6 years in the OTHERS group. The majority belonged to the less than 50 years age group in all the categories (62.4%) and were premenopausal (57.1%). Both the cases of UUS were postmenopausal. Abnormal uterine bleeding (AUB) was the foremost presenting symptom in majority of the groups (ULMS = 21.2%, LGESS = 15.3%, HGESS = 4.7%).
Ultrasonography was the major preoperative imaging modality identified in our study population (ULMS = 25.9%, LGESS = 16.5%, HGESS = 5.9%, OTHERS = 4.7%).
Table 1. Clinicopathologic characteristics of study population.
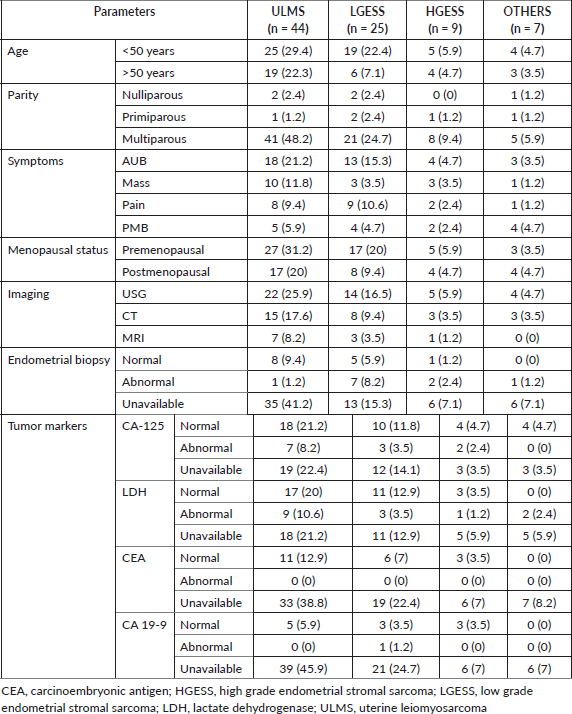
CA-125 levels were available only in 48 cases (56.5%) (25 cases of ULMS, 13 cases of LGESS, 6 cases of HGESS and 4 cases of OTHERS category) and was normal in majority (ULMS = 21.2%, LGESS = 11.8%, HGESS = 4.7%, OTHERS = 4.7%). LDH levels were available in 45 cases (52.9%) which included 26 cases of ULMS, 13 cases of LGESS, 4 cases of HGESS and 2 cases of OTHERS category. The majority had normal LDH levels except in the OTHERS category where LDH which was available in only two of the cases were abnormal (ULMS = 20%, LGESS = 12.9%, HGESS = 3.5%; OTHERS = 2.4%). CEA levels were available in 20 cases (23.5%) which included 11 cases of ULMS, 6 cases of LGESS and 3 cases of HGESS. All the cases had normal CEA levels. CA 19-9 levels were available in 12 cases (14.1%) which included 5 cases of ULMS, 4 cases of LGESS and 3 cases of HGESS. All the cases had normal CA 19-9 levels except for 1 case of LGESS which showed abnormal value.
Pathological characteristics are described in Table 2. The majority of cases were predominantly diagnosed in stage I (ULMS = 37.6%, LGESS = 16.5%, HGESS = 4.7%; OTHERS = 4.7%) followed by stage II (ULMS = 10.6%, LGESS = 9.4%, HGESS = 3.5%; OTHERS = 1.2%). Stage III presentation was seen in two (2.4%) cases each of ULMS and LGESS and one (1.2%) case each of HGESS and OTHERS category. There was one (1.2%) case each of stage IV in all the categories.
Tumour size of more than 5 cm was noted in all the subclasses except LGESS where the tumour size of less 5 cm was seen (ULMS = 50.6%, HGESS = 7%, OTHERS = 8.2%; LGESS = 18.8%,). An intramurally located tumour was noted in ULMS (36.5%) and HGESS (7%). Subserosal tumours were seen in LGESS (15.3%) and OTHERS (5.9%) categories.
The ER and PR status were available only for the cases of LGESS and HGESS, 17 and 5 cases, respectively. Both the hormone status was positive in 11 (12.9%) cases of LGESS and 2 (2.4%) cases of HGESS. ER alone was positive in 5 (5.9%) and 1 (1.2%) LGESS and HGESS cases, respectively. PR alone was positive in one (1.2%) case each of LGESS and HGESS, respectively.
Treatment
Surgical management with TAH with BSO was the primary mode of surgery in the majority in all the categories (ULMS = 31.8%, LGESS = 25.9%, HGESS = 5.9%; OTHERS = 4.7%). LND was done in 5 (5.9%) cases of ULMS, 1 (1.2%) case of LGESS and 2 (2.4%) cases each in HGESS and OTHERS categories. Omentectomy was done in six (7%) of ULMS, two (2.4%) cases of LGESS, four (4.7%) cases of HGESS and one (1.2%) case of OTHERS group. Adjuvant treatment was administered in 36 (42.5%) cases (Table 3). Radiotherapy in the form of Brachytherapy (8.2%) and EBRT (4.7%) were the major form rendered in ULMS. Hormonal treatment with letrozole was principally administered in LGESS (10.6%) and OTHERS category (2.4%). Chemotherapy with Docetaxel and Gemcitabine regimen was preferably administered in majority in HGESS (5.9%) (Table 4).
The median follow-up time was 37 months (Range 4–182 months). The overall recurrence rate in our study was 56.5%. To subclassify, it was seen in 28 (33%) cases of ULMS, 9 (36%) cases of LGESS, 7 (77.8%) cases of HGESS and 4 cases of the OTHERS category. AS patients had no recurrence.
There were three cases of AS of which two belonged to stage I and the other stage II. All three cases did not have a recurrence and are surviving till date. Thus, deriving a 5-year OS and DFS rate of 100%, respectively. Second, LGESS showed a better 5-year OS and DFS rate of 86.3% and 60.5%, respectively. The 5-year OS and DFS rates of ULMS were calculated to be 77.7% and 39.9%, respectively. The maximum follow-up of HGESS cases was 53 months (4.4 years). Hence the 3-year OS and DFS were calculated with values of 58.3% and 22.2%, respectively. The single cases of ERMS and ARMS in our study were of stage III and I, respectively. Both the cases had recurrence at 7 and 9 months, respectively. The two cases of UUS were of stage II and IV with recurrence at 11 and 6 months, respectively. All the above cases under the OTHERS category had succumbed to death depicting them as aggressive varieties. Kaplan Meier curves depicting OS and DFS are shown in Figures 1 and 2.
Prognostic variables on DFS and OS were studied using univariate analysis. Tumour size was found to be a significant factor affecting DFS of HGESS and OS of LGESS. LDH value was revealed to be affecting both DFS and OS of tumours under the OTHERS category. Stage of the tumour significantly affected the DFS and OS of LGESS, DFS of ULMS and OS of HGESS. The correlation of various clinicopathological factors affecting recurrence has revealed the significance of LDH value, the stage of the tumour and the nature of the adjuvant treatment (Table 5). Kaplan Meier curves demonstrating the clinicopathological features of LDH and stage of tumour affecting recurrence are shown in Figures 3 and 4.
Table 2. Details of final histopathological examination report.
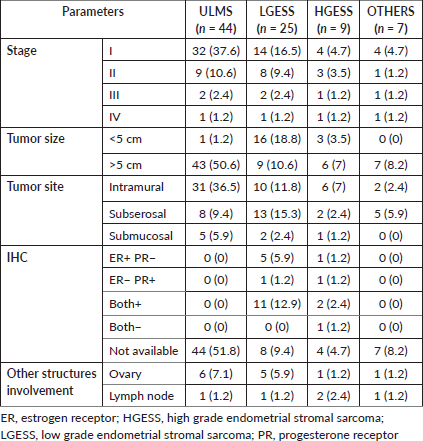
Table 3. Type of surgeries done in study population.
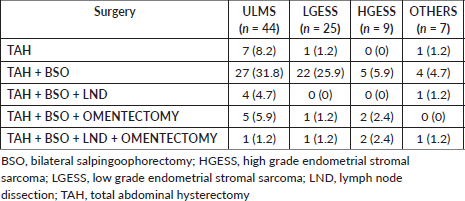
Table 4. Adjuvant treatment administered post-surgery.
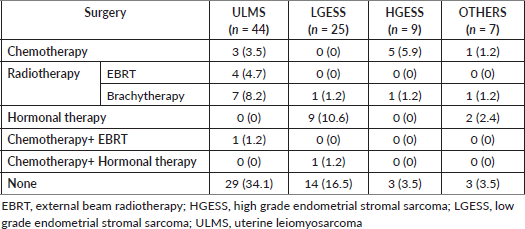
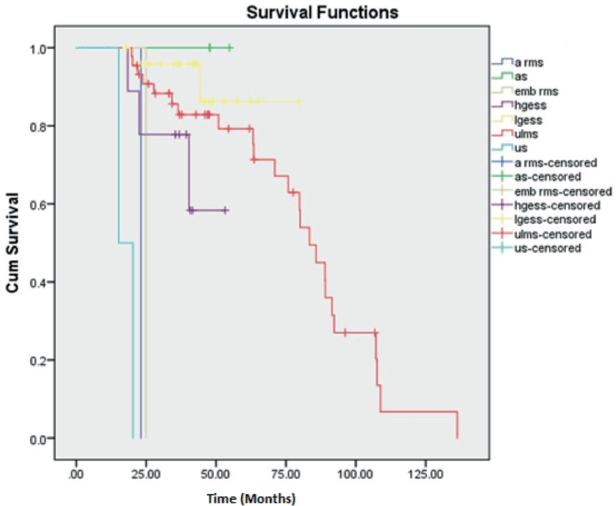
Figure 1. Kaplan Meier curve showing OS.
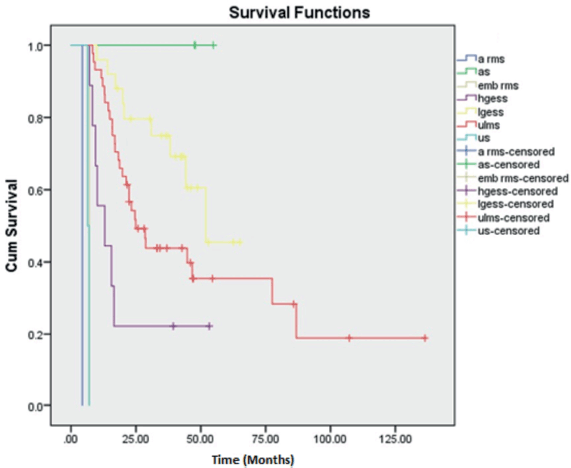
Figure 2. Kaplan Meier curve showing DFS.
Discussion
USs are rare gynaecological malignancies with histological diversities and higher recurrence rate therefore leading to inferior survival outcomes [2]. This 10-year retrospective study aimed to provide an understanding of the clinicopathological features and its associated prognostic factors.
ULMS was the common histopathological subtype (51.8%) identified in our study consistent with the previous reports [2, 8]. The age of presentation which varies for different histologies. In our study, the mean age of ULMS was 50.8 years. ESS cases showed a mean age of 45.5 and 48.1 years for low-grade and high-grade, respectively. These are similar to the results of the study by Wang et al [9]. Most of the patients in our study were premenopausal except for AS and UUS which were mostly postmenopausal. UUS cases had the highest average age of presentation. This is consistent with a multi-centric study by Li et al [10] where the cases were predominantly premenopausal and UUS cases had the oldest age of presentation.
AUB was the commonest presenting symptom, with PMB was seen in UUS and AS. Mass per abdomen and abdominal pain were the other significant symptoms noted, concordant with the findings by Thangappah et al [11]. The symptoms are probably due to the rapid-growing nature of these tumours. Hence any form of genital bleeding and rapidly enlarging masses should prompt suspicion of US. Preoperative endometrial sampling could aid in the diagnosis [12]. 12.9% of the cases of our study were preoperatively diagnosed by endometrial biopsy.
Table 5. Correlation of recurrence with clinicopathological features.
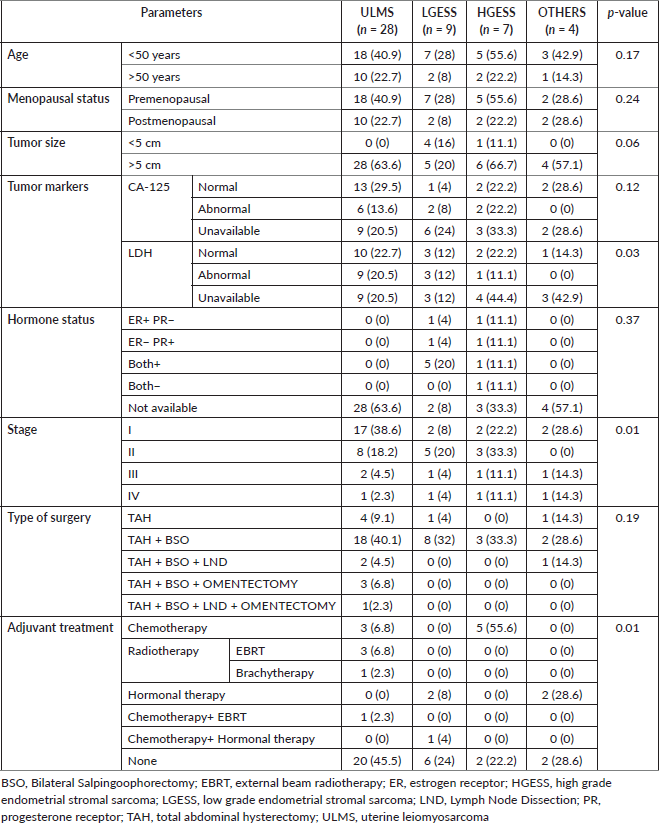
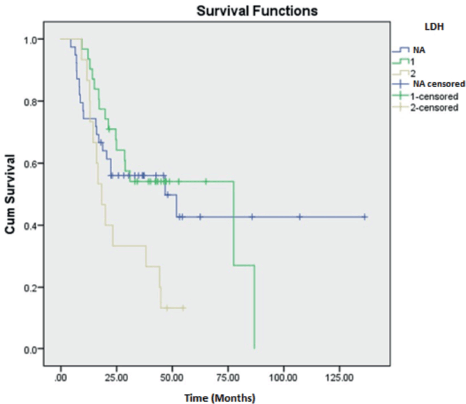
Figure 3. Kaplan Meier curve showing the effect of LDH value on recurrence.
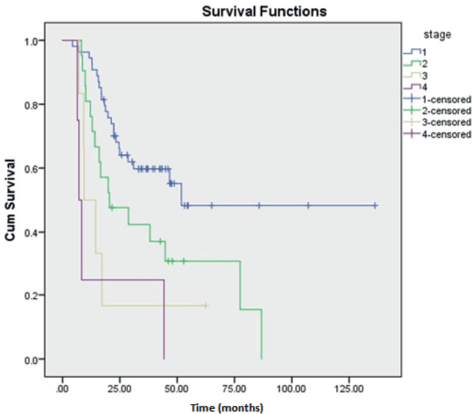
Figure 4. Kaplan Meier curve showing the effect of stage of the tumour on recurrence.
Ultrasound was the major imaging modality. Although recent literature suggests Magnetic resonance imaging with features such as irregular margins and inhomogeneous architecture with higher T2 signals are necessary in the workup [13, 14]. Cost remains a limiting factor in its implementation especially in financially low-performing nations.
The diagnostic accuracy of the tumour markers CA-125, LDH, CEA and CA 19-9 were assessed. CA-125 lacked significance, aligning with the findings by Yilmaz et al [15], though Duk et al [16] linked elevated levels to be a poor prognosis. Stimulated mesothelial cells are known to cause elevated CA-125 in US. LDH emerged as a significant prognostic variable for recurrence with Song et al [17] highlighting its relevance in ULMS. Our study showed significance of LDH for the tumours such AS, UUS and RMS in addition to ULMS. There are few case reports showing exceedingly higher LDH values in RMS cases although their significance has not been reported [18, 19]. As LDH is an enzyme involved in metabolism of the cancer cells, it could be elevated in many other conditions and cannot considered as a specific marker [20]. DiSaia et al [21] identified elevated CEA in a few cases of US. However, it was not found significant. The role of CA 19-9 secreted by the epithelial cells of uterus has only a minimal role in gynaecological malignancies [22]. This proclaims that none of the tumour markers are specific or sensitive for US.
ER and PR expression was highly demonstrated in LGESS compared to HGESS and ULMS. Davidson et al [23] reported ER and PR expression in 53% and 67% of the LGESS, 31% and 47% of HGESS and 45% and 65% of ULMS cases, respectively. This makes hormonal therapy a better adjuvant modality for LGESS [24]. Apart from ER and PR literatures suggest several other molecular markers for prognostication. These include JAZF1-SUZ12 gene fusion which is present in significant proportion of LGESS cases. This is associated with better outcome. CD10 is also a useful diagnostic marker of LGESS. YWHAE-NUTM2A/B fusion is a hallmark for HGESS. This along with BCOR alterations are associated with aggressive tumour behaviour. Ki-67, a proliferation marker is highly expressed in HGESS [5, 25]. Overexpression of MCM2 is often observed in ULMS and it helps to differentiate from leiomyomas [26]. Androgen receptor is used as a prognostic marker for ULMS [27]. Although these biomarkers have not been analysed in our study.
Most of the cases had presented at an earlier stage of stage I and II. Survival analysis showed the significance of tumour stage on DFS of ULMS and LGESS and OS of LGESS and HGESS comparable with the study by Cheng et al [28]. ULMS showed a little higher OS and an equivalent DFS as that of Ayhan et al [29] reporting 54% and 42%, respectively. LGESS outcomes were lower than Zhou et al [30] demonstrating a 5-year OS and DFS of 96.7% and 91.8%, respectively. A previous study reported a 3-year OS of 27.3% for HGESS which is much lower than our study [10]. A study on AS by Mancari et al [31] revealed a 5-year OS and DFS to be 89.5% and 85.3%, respectively, which is lower than in our study. All the differences might be due to the differences in the patient cohort and treatment modalities. LGESS and AS showed favourable survival outcomes in comparison with UUS and HGESS exhibiting poor survival outcomes. These are supported by a few previous studies [2, 9–11].
TAH with BSO was the primary surgical approach. The role of BSO in cases of ULMS remains controversial. Analysis by a few survival studies analysing the effect of ovarian preservation on survival revealed nil significance [32, 33]. Nevertheless, BSO is recommended for ESS cases as they are hormone-responsive tumours [6].
The evidence regarding the mode of adjuvant therapies in US is limited. Hao et al [34] studied 2,897 cases of ULMS and reported better survival in the radiotherapy group. This supports the findings of our study. Studies to evaluate the role of AI in LGESS suggest AI offer prolonged disease-free periods even in metastatic settings [35]. A case report by Altal et al [36] demonstrated complete remission of advanced LGESS with hormonal therapy with letrozole at the dose of 2.5 mg per day similar to the dosage followed in our study. Zhang et al [37] reported the benefits of the chemotherapeutic regimen adopted for HGESS identical to our study. However, studies do suggest Doxorubicin-based regimens as the preferred regimen in the treatment of US [38, 39]. In recurrent cases of ULMS, Trabectidin has shown efficacy [40]. Targeted therapies are recently becoming an interest particularly for tumours with high tumour mutational burden. Pembrolizumab, immune checkpoint inhibitor have been proven effective in microsatellite instability-high ULMS [41]. Pazopanib is another approved drug for US [42].
Recurrence remains a key concern though our study’s rate was lower than the 53%–71% reported by Giuntoli et al [43], likely due to larger patient cohort in earlier stages than the advanced stages. The relapse rate of ESS ranges from 36% to 56% coinciding with that of LGESS. However, an increased recurrence rate was noted in HGESS in our study [44]. AS are reported to show a long latent course with good survival which is comparable with the cases of our study [45]. Tumour stage and size influenced survival and recurrence [2].
US is a rare tumour with a larger number of only retrospective studies in literature [2, 9–11]. Further prospective studies are essential to develop a definitive management protocol. The advantage of our study is that, this is an oncology-dedicated tertiary-care centre study. However, there are certain limitations which include its retrospective nature with smaller number of cases of subtypes like AS, UUS and RMS from a single centre. This led to the inability of a comprehensive subgroup analysis of all varieties separately.
Conclusion
US tends to recur with prognosis varying among the subtypes. Our study revealed that AS and LGESS show better survival outcomes. Prognostic factors for recurrence were LDH value, stage of the tumour and nature of the adjuvant treatment. A prompt diagnosis at an earlier stage with a proper workup and timely intervention including its adjuvant treatment would help to mitigate the severity of the disease. A vigilant follow-up is recommended to pick up the recurrences beforehand.
Conflicts of interest
None.
Funding
No funding was received for the study.
Author contributions
All the authors contributed to the conception of the work, preparation of manuscript and revision of the manuscript critically for intellectual content.
References
1. Giannini A, Golia D'augè T, and Bogani G, et al (2023) Uterine sarcomas: a critical review of the literature Eur J Obstet Gynecol Reprod Biol 287 166–170 https://doi.org/10.1016/j.ejogrb.2023.06.016 PMID: 37348383
2. Wang F, Dai X, and Chen H, et al (2022) Clinical characteristics and prognosis analysis of uterine sarcoma: a single-institution retrospective study BMC Cancer 22(1) 1050 https://doi.org/10.1186/s12885-022-10129-x PMID: 36207687 PMCID: 9540718
3. Skorstad M, Kent A, and Lieng M (2016) Uterine leiomyosarcoma – incidence, treatment, and the impact of morcellation. A nationwide cohort study Acta Obstet Gynecol Scand 95(9) 984–990 https://doi.org/10.1111/aogs.12930 PMID: 27223683
4. Harlow BL, Weiss NS, and Lofton S (1986) The epidemiology of sarcomas of the uterus J Natl Cancer Inst 76(3) 399–402 PMID: 3456457
5. Ferrandina G, Aristei C, and Biondetti PR, et al (2020) Italian consensus conference on management of uterine sarcomas on behalf of S.I.G.O. (Societa’ italiana di Ginecologia E Ostetricia) Eur J Cancer 139 149–168 https://doi.org/10.1016/j.ejca.2020.08.016 PMID: 32992154
6. Mbatani N, Olawaiye AB, and Prat J (2018) Uterine sarcomas Int J Gynecol Obstet 143(S2) 51–58 https://doi.org/10.1002/ijgo.12613
7. Cantú De León D, González H, and Pérez Montiel D, et al (2013) Uterine sarcomas: review of 26 years at The Instituto Nacional de Cancerologia of Mexico Int J Surg 11(7) 518–523 https://doi.org/10.1016/j.ijsu.2013.04.013 PMID: 23664822
8. Barquet-Muñoz SA, Isla-Ortiz D, and Montalvo-Esquivel G, et al (2019) Prognostic factors associated with uterine sarcomas: the experience of a single institution J Obstet Gynaecol J Inst Obstet Gynaecol 39(2) 231–236 https://doi.org/10.1080/01443615.2018.1492529
9. Wang JF, Li C, and Yang JY, et al (2024) Clinicopathological characteristics and prognosis of uterine sarcoma: a 10-year retrospective single-center study in China Diagn Pathol 19(1) 94 https://doi.org/10.1186/s13000-024-01517-x PMID: 38970112 PMCID: 11225383
10. Li D, Yin N, and Du G, et al (2020) A real-world study on diagnosis and treatment of uterine sarcoma in western China Int J Biol Sci 16(3) 388–395 https://doi.org/10.7150/ijbs.39773 PMID: 32015676 PMCID: 6990907
11. Thangappah RBP (2019) Uterine sarcoma: a clinico-pathological study J Obstet Gynaecol India 69(Suppl 2) 147–152 https://doi.org/10.1007/s13224-018-1141-5 PMID: 31686748 PMCID: 6801271
12. Dijkhuizen FPHLJ, Mol BWJ, and BröLmann HAM, et al (2000) The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis Cancer 89(8) 1765–1772 https://doi.org/10.1002/1097-0142(20001015)89:8<1765::AID-CNCR17>3.0.CO;2-F PMID: 11042572
13. Hindman N, Kang S, and Fournier L, et al (2023) MRI evaluation of uterine masses for risk of leiomyosarcoma: a consensus statement Radiology 306(2) e211658 https://doi.org/10.1148/radiol.211658
14. Valletta R, Corato V, and Lombardo F, et al (2024) Leiomyoma or sarcoma? MRI performance in the differential diagnosis of sonographically suspicious uterine masses Eur J Radiol 170 https://doi.org/10.1016/j.ejrad.2023.111217
15. Yilmaz N, Sahin I, and Kilic S, et al (2009) Assessment of the predictivity of preoperative serum CA 125 in the differential diagnosis of uterine leiomyoma and uterine sarcoma in the Turkish female population Eur J Gynaecol Oncol 30(4) 412–414 PMID: 19761133
16. Duk JM, Bouma J, and Burger GTN, et al (1994) CA 125 in serum and tumor from patients with uterine sarcoma Int J Gynecol Cancer 4(3) https://doi.org/10.1046/j.1525-1438.1994.04030156.x PMID: 11578400
17. Song K juan, Yu X ni, and Lv T, et al (2018) Expression and prognostic value of lactate dehydrogenase-A and -D subunits in human uterine myoma and uterine sarcoma Med (Baltimore) 97(14) 268
18. Tamura S, Hayashi T, and Ichimura T, et al (2022) Characteristic of uterine rhabdomyosarcoma by algorithm of potential biomarkers for uterine mesenchymal tumor Curr Oncol 29(4) 2350–2363 https://doi.org/10.3390/curroncol29040190 PMID: 35448164 PMCID: 9027675
19. Li ZJ, Li CL, and Wang W, et al (2021) Diagnosis and treatment of pleomorphic rhabdomyosarcoma of the uterus: a rare case report and review of the literature J Int Med Res 49(5) 03000605211014360 https://doi.org/10.1177/03000605211014360 PMCID: 8161909
20. Suh DS, Song YJ, and Roh HJ, et al (2021) Preoperative blood inflammatory markers for the differentiation of uterine leiomyosarcoma from leiomyoma Cancer Manag Res 13 5001–5011 https://doi.org/10.2147/CMAR.S314219 PMID: 34211296 PMCID: 8239166
21. Disaia PJ, Haverback BJ, and Dyce BJ, et al (1975) Carcinoembryonic antigen in patients with gynecologic malignancies Am J Obstet Gynecol 121(2) 159–163 https://doi.org/10.1016/0002-9378(75)90631-6 PMID: 163585
22. Lee T, Teng TZJ, and Shelat VG (2020) Carbohydrate antigen 19-9 — tumor marker: past, present, and future World J Gastrointest Surg 12(12) 468–490 https://doi.org/10.4240/wjgs.v12.i12.468
23. Davidson B, Kjæreng ML, and Førsund M, et al (2016) Progesterone receptor expression is an independent prognosticator in FIGO stage I uterine leiomyosarcoma Am J Clin Pathol 145(4) 449–458 https://doi.org/10.1093/ajcp/aqw030 PMID: 27149024
24. Maccaroni E, Lunerti V, and Agostinelli V, et al (2022) New insights into hormonal therapies in uterine sarcomas Cancers 14(4) 921 https://doi.org/10.3390/cancers14040921 PMID: 35205669 PMCID: 8870116
25. Pérez-Fidalgo JA, Ortega E, and Ponce J, et al (2023) Uterine sarcomas: clinical practice guidelines for diagnosis, treatment, and follow-up, by Spanish group for research on sarcomas (GEIS) Ther Adv Med Oncol 15 17588359231157645 https://doi.org/10.1177/17588359231157645 PMID: 37007636 PMCID: 10052607
26. Guo J, Zheng J, and Tong J (2024) Potential markers to differentiate uterine leiomyosarcomas from leiomyomas Int J Med Sci 21(7) 1227–1240 https://doi.org/10.7150/ijms.93464 PMID: 38818470 PMCID: 11134592
27. Baek MH, Park JY, and Park Y, et al (2018) Androgen receptor as a prognostic biomarker and therapeutic target in uterine leiomyosarcoma J Gynecol Oncol 29(3) https://doi.org/10.3802/jgo.2018.29.e30
28. Cheng G, Li Y, and Qu P (2020) Clinicopathological characteristics of patients with uterine sarcoma: clinical presentation, treatment, and survival outcomes Int J Clin Exp Med 13(10) 7794–7804
29. Ayhan A, Gungorduk K, and Khatib G, et al (2021) Prognostic factors and survival outcomes of women with uterine leiomyosarcoma: a Turkish Uterine Sarcoma Group Study-003 Curr Probl Cancer 45(5) 100712 https://doi.org/10.1016/j.currproblcancer.2021.100712 PMID: 33685725
30. Zhou J, Zheng H, and Wu SG, et al (2015) Influence of different treatment modalities on survival of patients with low-grade endometrial stromal sarcoma: a retrospective cohort study Int J Surg 23 147–151 https://doi.org/10.1016/j.ijsu.2015.09.072 PMID: 26449652
31. Mancari R, Yusuf Y, and Macuks R, et al (2024) Prognostic factors in uterine adenosarcoma: subanalysis of the SARCUT study Front Oncol 14 1393707 https://doi.org/10.3389/fonc.2024.1393707 PMID: 38835369 PMCID: 11148341
32. Giuntoli RL, Metzinger DS, and Dimarco CS, et al (2003) Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy Gynecol Oncol 89(3) 460–469 https://doi.org/10.1016/S0090-8258(03)00137-9 PMID: 12798712
33. Gadducci A, Landoni F, and Sartori E, et al (1996) Uterine Leiomyosarcoma: analysis of Treatment Failures and Survival Gynecol Oncol 62(1) 25–32 https://doi.org/10.1006/gyno.1996.0185 PMID: 8690287
34. Hao Z and Yang S (2022) The role of postoperative radiotherapy in patients with uterine sarcomas: a PSM-IPTW analysis based on SEER database Front Surg 9 985654 https://doi.org/10.3389/fsurg.2022.985654 PMID: 36017510 PMCID: 9395938
35. Crowley F, Cadoo KA, and Chiang S, et al (2022) Evaluating the role of aromatase inhibitors in the treatment of low-grade endometrial stromal sarcomas Gynecol Oncol Rep 40 100980 https://doi.org/10.1016/j.gore.2022.100980 PMID: 35434239 PMCID: 9011013
36. Altal OF, Al Sharie AH, and Halalsheh OM, et al (2021) Complete remission of advanced low-grade endometrial stromal sarcoma after aromatase inhibitor therapy: a case report J Med Case Rep 15(1) 262 https://doi.org/10.1186/s13256-021-02838-x PMID: 33947445 PMCID: 8097811
37. Zhang Y, Chen C, and Ren M, et al (2019) Treatment of uterine high-grade endometrial stromal sarcoma with apatinib combined with chemotherapy Med (Baltimore) 98(13) e15050 https://doi.org/10.1097/MD.0000000000015050
38. Turinetto M, Meeus P, and Ray-Coquard I (2023) Doxorubicin combined with Trabectedin in systemic first-line M+/recurrent leiomyosarcoma patients Curr Opin Oncol 35(4) 288 https://doi.org/10.1097/CCO.0000000000000961 PMID: 37222199
39. Kim T, Hao C, and Pan M, et al (2025) Gemcitabine plus docetaxel, dacarbazine, doxorubicin combinations, or doxorubicin alone as first-line treatment for advanced/metastatic leiomyosarcoma: a retrospective analysis at a sarcoma center Diseases 13(3) 79 https://doi.org/10.3390/diseases13030079 PMID: 40136620 PMCID: 11941542
40. Monk BJ, Blessing JA, and Street DG, et al (2012) A phase II evaluation of trabectedin in the treatment of advanced, persistent, or recurrent uterine leiomyosarcoma: a gynecologic oncology group study Gynecol Oncol 124(1) 48–52 https://doi.org/10.1016/j.ygyno.2011.09.019
41. Wang YJ, Williams HR, and Brzezinska BN, et al (2021) Use of pembrolizumab in MSI-high uterine leiomyosarcoma; a case report and review of the literature Gynecol Oncol Rep 35 100701 https://doi.org/10.1016/j.gore.2021.100701 PMID: 33537390 PMCID: 7843391
42. Cuppens T, Tuyaerts S, and Amant F (2015) Potential therapeutic targets in uterine sarcomas Sarcoma 2015 243298 https://doi.org/10.1155/2015/243298 PMID: 26576131 PMCID: 4632006
43. Giuntoli RL, Garrett-Mayer E, and Bristow RE, et al (2007) Secondary cytoreduction in the management of recurrent uterine leiomyosarcoma Gynecol Oncol 106(1) 82–88 https://doi.org/10.1016/j.ygyno.2007.02.031 PMID: 17434579
44. Amant F, Coosemans A, and Debiec-Rychter M, et al (2009) Clinical management of uterine sarcomas Lancet Oncol 10(12) 1188–1198 https://doi.org/10.1016/S1470-2045(09)70226-8 PMID: 19959075
45. Korets SB and Curtin JP (2012) Surgical options for recurrent uterine sarcomas Am Soc Clin Oncol Educ Book 32 362–366 https://doi.org/10.14694/EdBook_AM.2012.32.70





