Low incidence of primary immunodeficiency-associated cancers in children at a tertiary care pediatric hospital in Pakistan: a blessing in disguise or wet behind the ears?
Rahat Ul Ain and Mahwish Faizan
Department of Pediatric Hematology/Oncology and Bone Marrow Transplant, University of Child Health Sciences, The Children’s Hospital, Lahore 54600, Pakistan
Abstract
Scarce data is available regarding primary immunodeficiency-associated cancers in children in low-middle-income countries. This study aimed to determine the incidence, clinical features and outcomes of primary immunodeficiencies (PIDs)-associated cancers in children presenting to Pakistan’s largest public-sector specialised pediatric oncology center. Among 5,748 children with cancers registered over 5 years, only eight patients were found to have PID-associated pediatric malignancies with an incidence of 1.4 per 1,000 cases. The median age at the time of diagnosis was 6.5 years with a male-to-female ratio of 7:1. Only four types of PIDs were found to be associated with cancer in children at our center: Ataxia Telangiectasia in 37.5% (n = 3), hyper-IgE syndrome and IgG deficiency in 25% (each n = 2) and one case (12.5%) of common variable immune deficiency. Six different types of pediatric cancers were associated with PID with a predisposition towards hematological malignancies (n = 7, 87.5%). Only two patients (25%) survived. The median survival of the cohort was 3.5 months. Infection-related mortality was the cause of death in four patients (66%), and the type of PID was the only statistically significant factor associated with the outcome. It is concluded that a lesser proportion of PID-associated pediatric cancers are found in our center as compared to the reported data from high-income countries. PID-associated cancers in children have an abysmal prognosis and infection-related mortality is the major cause of treatment failure. Sensitisation of oncologists to look for any underlying PID, the introduction of PID-screening programs in children and consideration of PID-associated malignancies as a high-risk group for treatment may help improve the outcomes.
Keywords: incidence, immune deficiency, pediatric oncology, cancers, children, low-middle-income countries
Correspondence to: Rahat Ul Ain
Email: dr.rkashif@yahoo.com
Published: 30/07/2024
Received: 04/03/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Primary immunodeficiencies (PIDs) also known as inborn errors of immunity (IEIs) are a group of inherited immune system disorders associated with increased susceptibility to infections, autoimmunity, allergy and cancer [1]. More than 250 different types of PIDs/IEIs have been described so far [2]. The failure in the regulation of the immune system leads to multiple diseases due to the absence of cytokines, impaired function of the B or T lymphocytes, lack of functioning proteins, or alteration in receptors [3]. Tumour cell antigens induce immune responses that destroy tumour cells developed in the body therefore the immunological approach is being increasingly used not only for the diagnosis but also in the treatment of cancer [4]. Children with primary immunodeficiency have an estimated overall risk of 4%–25% of developing malignancy [5]; therefore, it is important to recognise this condition but minimal data is available from developing countries in this regard. This study aimed to determine the incidence, clinical features and outcomes of PID-associated cancers in children presenting to Pakistan’s largest public-sector specialised pediatric oncology center.
Methods
An ambi-directional cohort study was conducted in the Department of Pediatric Hematology/Oncology at the University of Child Health Sciences, The Children’s Hospital Lahore, Pakistan. After the approval from the institutional ethical committee, the data were collected retrospectively from January 2017 to August 2020 and prospectively from September 2020 to December 2021, with a total period of 5 years. The study included all the children with pediatric malignancies diagnosed with underlying primary immunodeficiency, aged less than 16 years. Patients with Down syndrome, Fanconi anemia and Histiocytosis syndromes were excluded. After the completion of data collection in December 2021, the study cohort was followed up for 2 years till December 2023 for survival analysis. The data were analysed in terms of descriptive statistics with SPSS version 20.0. The survival analysis was done using the Kaplan–Meier and log-rank tests. The chi-square test was applied for the determination of p-values.
Primary Immunodeficiency: Patients with primary immunodeficiency were defined as having a clinical diagnosis of one of the genetic syndromes associated with innate immune system defects.
Results
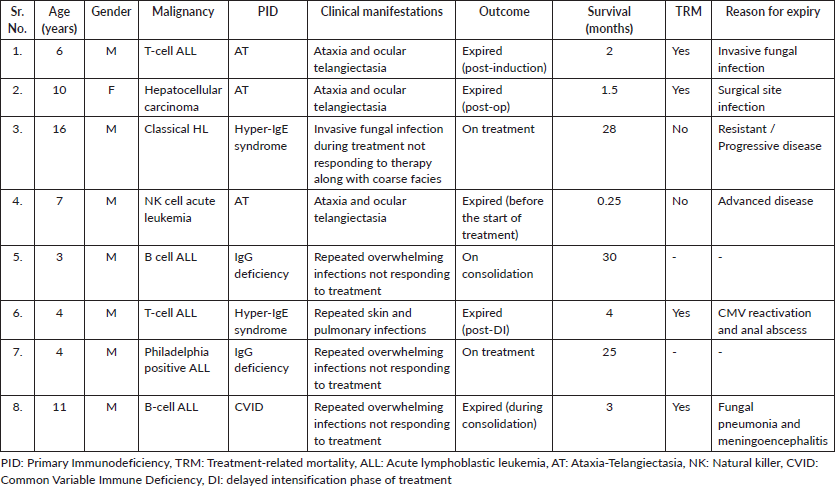
A total of 5,748 pediatric patients with different malignant disorders were registered in the department over the 5 years study period (From January 2017 till December 2021), while only 8 patients were found to have an underlying PID with an incidence of 1.4 cases/1,000 pediatric cancer cases. The median age of presentation of patients with PID-associated malignancies was 6.5 years (3–16 years) with the male to female ratio of 7:1.
Only four types of PIDs were found to be associated with cancer in children at our center: Ataxia Telangiectasia (AT) in 37.5% (n = 3), Hyper-IgE syndrome and IgG deficiency in 25% each (n = 2 each) and one case (12.5%) of common variable immune deficiency (CVID). Similarly, six different types of malignant disorders were found to be associated with children with PID: B-cell acute lymphoblastic leukemia (ALL) (25%, n = 2), T-cell ALL (25%, n = 2), Philadelphia positive ALL (12.5%, n = 1), NK-cell ALL (12.5%, n = 1), Hodgkin lymphoma (HL) (12.5%, n = 1) and hepatocellular carcinoma (12.5%, n = 1) (Table 1).
The median survival was 3.5 months (0.25–30 months) with a survival rate of 25% (n = 2, one patient completed treatment and one is under treatment) (Figure 1). The type of PID was the only significant factor associated with the outcome (p-value: 0.046).
Discussion
The most common PID, CVID is associated with an increased risk of B-cell non-HL and gastric cancer but these rarely develop in childhood. Similarly, hyper-IgE syndrome also has a higher risk of developing aggressive B cell lymphomas. Chronic mucocutaneous candidiasis is associated with squamous cell cancers, and AT with the highest risk for malignancy among all PID, leukemia and lymphomas is frequently observed [6].
Table 1. Characteristics of the children with PID-associated cancers.
As compared to the published literature, mainly from the high-income countries (HICs) [3, 5, 7–10], a strikingly lesser proportion of PID-associated pediatric malignancies is found in this study. This either can be due to a lesser incidence of PID in our region or a lack of diagnosis of all PID cases. The former seems to be less likely as our country has a high rate of consanguinity and it is expected to have a higher proportion of all autosomal recessive disorders in the population, while the latter seems to be more likely the cause of this finding in our setting. There could be multiple reasons behind the lack of diagnosis of all cases of PID among the children presenting with cancer in our hospital. In this study, though a large number of pediatric oncology patients were registered during the study period, all of these patients did not go through any screening test for immune deficiency on presentation with cancer. Previous history of repeated infections was not taken into the record and definitive diagnostic tests (genetic testing) for primary immunodeficiency are not available in our setting. PIDs were rather diagnosed later during treatment in all patients except one who had a known case of AT. The diagnosis of PID was based on the recognition of specific clinical features (phenotypic abnormalities) of various immune deficiency syndromes or the development of resistant/overwhelming infections during treatment. Further diagnosis was augmented with the immunoglobulin levels. As the tests for immunoglobulin levels were done post-chemotherapy in the majority of the cases; therefore, it was made sure to have bone marrow recovery with a normal white cell count, absolute neutrophil count and absolute lymphocyte count while checking the sample for immunoglobulin levels. There is also a lack of screening programs at birth for genetic disorders in our country so despite having a large number of cases with pediatric cancers, many cases of PID would have been missed being included in the study and may lead to a smaller number of reported cases of PID-associated pediatric malignancies at our center.
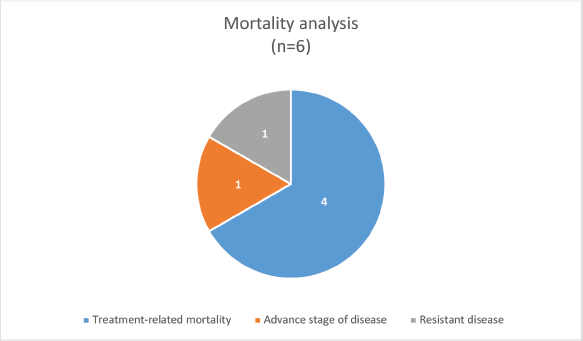
Figure 1. Mortality analysis of PID-associated pediatric cancers.
A different clinical spectrum of PID-associated pediatric malignancies is also noted in this study. As compared to the previous studies [3, 5, 7–9] from HIC, the types of PIDs, types of associated cancers and the median age of presentation all are different. Non-HL has been reported to be the most common malignancy, and CVID is the most common PID with a median age of 7.1 years on presentation. Filipovich et al [7] found male predominance, contributed to several X-linked PIDs as well as the surprising observation of excess tumours in males with AT, an autosomal recessive disorder. In this study, male preponderance has also been observed but none of the X-linked PIDs have been found, and the patients with AT did not show any sex predilection. The possible explanation for these distinctive findings and probably the limitations to this study are that this study has been conducted among oncology patients in the pediatric age group (<16 years) only, with a small number of PID cases recognised in the study cohort resulting in a very small sample size to be analysed for. Future studies with a larger cohort and improved diagnostics for PIDs should be conducted from our region.
The outcome of PID-associated pediatric cancers in the setting of a low-middle income country (LMIC) is poor with a substantial number of patients having infection/treatment-related mortality. Primary immunodeficiency with chemotherapy-induced secondary immunodeficiency, along with the underlying canvas of suboptimal supportive care in LMIC settings adds up to these challenges. To combat these challenges in resource-limited settings it is suggested that this group of patients should be considered as a high-risk group during risk stratification with consideration of vigilant supportive care during treatment. This can be done in the form of enhanced infection prevention and control measures, and extended infection chemoprophylaxis (including not only against pneumocystis jiroveci pneumonia, but also antifungal antiviral, and antibiotic prophylaxis). Similarly, granulocyte colony-stimulating factors and intravenous immunoglobulin should be considered among the first-line therapy in the management of febrile neutropenia.
Management of children with a combination of PID and cancer is particularly challenging in a resource-limited setting where there are suboptimal healthcare facilities and the absence of screening programs for genetic disorders at birth. A substantial number of genetic disorders remain undiagnosed and present later on in life with various complications. Timely diagnosis of these disorders can not only benefit in terms of the patient’s outcomes but also provide the opportunity to screen close family members and offer appropriate management and surveillance. The oncologists working in LMIC should at least make a habit of taking the previous history of repeated infections, family history and evaluation of baseline immunoglobulin levels, in patients presenting with malignant disorders. These measures are cost-effective and feasible in resource-limited settings and can help diagnose, not all, but a substantial number of patients with cancers with PIDs.
Conclusion
A lesser proportion of PID-associated pediatric malignancies have been found as compared to the data from HICs. Either primary immunodeficiency rarely presents with cancers in childhood in our region or it is under-diagnosed in resource-limited settings, it is yet to be investigated further. PID-associated pediatric cancers have an abysmal prognosis and infection-related mortality is the major cause of treatment failure. Sensitisation of pediatric oncologists regarding baseline assessment for PIDs in all children with cancer, the introduction of PID-screening programs in children in the country, and consideration of PID-associated pediatric malignancies as a high-risk group for treatment may help improve the outcomes.
List of abbreviations
ALL, Acute lymphoblastic leukemia; AT, Ataxia-Telangiectasia; CVID, Common variable immune deficiency; HL, Hodgkin lymphoma; LMIC, Low/middle-income country; PID, Primary Immunodeficiency; TRM, Treatment-related mortality.
Acknowledgments
Aiza Kashif Malik for helping us with data collection.
Conflicts of interest
The authors have no potential conflicts of interest to disclose.
Funding
The authors have not received any funding for this study.
Author contributions
RUA: Conception, data collection, data analysis and manuscript writing.
MF: Data analysis and manuscript writing.
References
1. Akalu YT and Bogunovic D (2024) Inborn errors of immunity: an expanding universe of disease and genetic architecture Nat Rev Genet 25 184–195 https://doi.org/10.1038/s41576-023-00656-z
2. Picard C, Al-Herz W, and Bousfiha A, et al (2015) Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015 J Clin Immunol 35(8) 696–726 https://doi.org/10.1007/s10875-015-0201-1 PMID: 26482257 PMCID: 4659841
3. Naidoo R, Ungerer L, and Cooper M, et al (2011) Primary immunodeficiencies: a 27-year review at a tertiary paediatric hospital in Cape Town, South Africa J Clin Immunol 31 99–105 https://doi.org/10.1007/s10875-010-9465-7
4. Martin-Mateos MA and Gilbert MP (2016) Primary immunodeficiencies and B-cell lymphomas Bol Med Hosp Infant Mex 73(1) 18–25 PMID: 29421227
5. Mueller BU and Pizzo PA (1996) Cancer in children with primary or secondary immunodeficiencies J Pediatr 126 1–10 https://doi.org/10.1016/S0022-3476(95)70491-4
6. Mortaz E, Tabarsi P, and Mansouri D, et al (2016) Cancers related to immunodeficiencies: update and perspectives Front Immunol 7 365 https://doi.org/10.3389/fimmu.2016.00365 PMID: 27703456 PMCID: 5028721
7. Filipovich AH, Mathur A, and Kamat D, et al (1992) Primary immunodeficiencies: genetic risk factors for lymphoma Cancer Res 52 5465–5467
8. Frizzera G, Rosai J, and Dehner LP, et al (1980) Lymphoreticular disorders in primary immunodeficiencies: new findings based on an up-to-date histologic classification of 35 cases Cancer 15 692–699 3.0.CO;2-Q>https://doi.org/10.1002/1097-0142(19800815)46:4<692::AID-CNCR2820460410>3.0.CO;2-Q
9. Mayor PC, Eng KH, and Singel KL, et al (2018) Cancer in primary immunodeficiency diseases: cancer incidence in the United States Immune Deficiency Network Registry J Allergy Clin Immunol 141(3) 1028–1035 https://doi.org/10.1016/j.jaci.2017.05.024
10. Kebudi R, Kiykim A, and Sahin MK (2019) Primary immunodeficiency and cancer in children; a review of the literature Curr Pediatr Rev 15(4) 245–250 https://doi.org/10.2174/1573396315666190917154058 PMID: 31530267 PMCID: 7040504






