Association between pre-existing cardiometabolic comorbidities and the pathological profiles of breast cancer at initial diagnosis: a cross sectional study
Shridevi Subramaniam1, Yek-Ching Kong2, Cheng-Har Yip3, Muthukkumaran Thiagarajan4, Jayalakshmi Pailoor3, Hafizah Zaharah5, Nur Aishah Taib6, Mee-Hoong See6, Diana Sarfati7 and Nirmala Bhoo-Pathy2
1Centre for Clinical Epidemiology, Institute for Clinical Research, National Institutes of Health, Ministry of Health, Shah Alam 40170, Malaysia
2Centre for Epidemiology and Evidence-Based Medicine, Department of Social and Preventive Medicine, Faculty of Medicine, Universiti Malaya, Kuala Lumpur 50603, Malaysia
3Subang Jaya Medical Centre, Subang Jaya 47500, Malaysia
4Department of Radiotherapy and Oncology, Kuala Lumpur Hospital, Ministry of Health, Kuala Lumpur 50586, Malaysia
5Department of Radiotherapy and Oncology, National Cancer Institute, Ministry of Health, Putrajaya 62250, Malaysia
6Department of Surgery, Faculty of Medicine, Universiti Malaya, Kuala Lumpur 50603, Malaysia
7National Director of Cancer Control, and Chief Executive Cancer Control Agency, PO Box 5013, Wellington 6140, New Zealand
Abstract
The presence of comorbidities has been associated with later stages of breast cancer diagnosis. It is unclear whether biological mechanisms are partly responsible. We examined the association between the presence of pre-existing comorbidities and tumour profile at initial diagnosis with breast cancer. Data for the present analysis were derived from a prior inception cohort study comprising 2,501 multiethnic women, newly diagnosed with breast cancer between 2015 and 2017 in four hospitals across Klang Valley. At the inception of the cohort, medical and drug histories, height, weight and blood pressure were recorded. Blood samples were taken to measure serum lipid and glucose. Modified Charlson Comorbidity Index (CCI) was calculated using data extracted from medical records. The association of CCI as well as specific comorbidities, with pathological breast cancer profile was analysed. Higher comorbidity burden, namely cardiometabolic conditions were associated with unfavourable pathological features including larger tumours, involvement of >9 axillary lymph nodes, distant metastasis and human epidermal growth factor receptor 2 overexpression. These associations remained largely significant following multivariable analyses. Specifically, diabetes mellitus was independently associated with high nodal metastasis burden. Low level of high-density lipoprotein was associated with larger tumours (>5 cm), and distant metastasis. Evidence from this study seems to support the hypothesis that the later stages of breast cancer diagnosis in women with (cardiometabolic) comorbidities may be partially explained by underlying pathophysiological events.
Keywords: cancer, comorbidity, cardiometabolic, cardiovascular disease, pathology
Correspondence to: Nirmala Bhoo-Pathy
Email: ovenjjay@gmail.com and nirmala.bhoopathy@ummc.edu.my
Published: 23/02/2023
Received: 27/09/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Comorbidity is increasingly being recognised as an important prognostic factor following breast cancer, where it has been associated with adverse clinical outcomes and patient-centred outcomes [1–4]. Prior literature suggest that comorbidity may influence cancer stage at diagnosis both positively and negatively [5]. For instance, as cancer and common comorbidities share many similar risk factors and symptoms, any early cancer symptoms may be brushed off or misinterpreted as symptoms of the pre-existing health condition, leading to delayed cancer diagnosis and subsequently, more advanced cancer stages at diagnosis [6]. Conversely, frequent clinical encounters for management of comorbidity can also lead to earlier cancer diagnosis among those with pre-existing illness [7].
From a biological perspective, the presence of several overlapping pathophysiological mechanisms between common comorbidities and cancer may directly or indirectly impact stage at cancer diagnosis [8]. This is corroborated by evidence suggesting that the presence of comorbidity may result in local and systemic inflammation leading to alterations in the tumour microenvironment, which can exacerbate cell proliferation and carcinogenesis [9–11]. If biological mechanisms do indeed at least partly explain the association between comorbidities and stage at diagnosis in breast cancer, it is postulated that there will also be differences in distribution of its pathologic prognostic factors by comorbidity status. Additionally, such associations may be specific to certain types or categories of comorbid conditions such as the association between diabetes mellitus and cancer metastasis, which can be explained by the high circulating levels of insulin and insulin-like growth factors in people with diabetes [10].
Nonetheless, prior research examining the overlap between cancer and common comorbidities such as cardiovascular diseases appear to have largely focused on shared risk factors [12]. While most of the current evidence seems to imply that the association between comorbidity and cancer stage at diagnosis is largely explained by non-biological factors such as patient’s health behaviour, physician’s attitude and delivery of preventive care, it remains unclear whether the association may also be explained by biological factors. In this study, we had hypothesised that if biological mechanisms do indeed at least partly explain the association between comorbidities and stage at diagnosis in breast cancer, there will also be differences in the distribution of its pathologic prognostic factors by comorbidity status. We undertook a cross-sectional analysis of an inception cohort study to examine the association between pre-existing common comorbidities and tumour profile at initial breast cancer diagnosis. Specifically, we aimed to gain insights on whether there is evidence of unfavourable pathological features (tumour histology, pathological tumour size, nodal involvement, distant metastasis, tumour grade, hormone receptor status, human epidermal growth factor receptor 2 (HER2) status) among women with common comorbid conditions.
Methods
Study population
Data for the present analysis were derived from a prior inception cohort study, which recruited women newly diagnosed with breast cancer between 2015 and 2017 in four hospitals across Klang Valley, an urban conglomeration in Malaysia [13]. The sample for this study was drawn from various public and private hospitals in Malaysia; National Cancer Institute (national oncology referral centre), Kuala Lumpur Hospital (public general hospital), University Malaya Medical Centre (public university hospital) and Subang Jaya Medical Centre (private medical centre). This was expected to allow the recruitment of a diverse sample of Malaysians with breast cancer from various ethnic and socioeconomic backgrounds, which in turn are predictors of cardiovascular risk factors and other pre-existing comorbidities [14]. Patients with recurrent cancers were excluded.
Study variables
Participants were recruited into the main study during their hospital visits. Demographic data including ethnicity (Malay, Chinese, Indian or other ethnicity), and age at diagnosis, as well as data on pre-existing medical conditions were collected via face-to-face interviews.
During the on-site screenings, height and body weight were measured. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Obesity was recorded if the calculated BMI was 30 kg/m2 and above. Systolic and diastolic blood pressure (BP) readings were taken using an automated BP monitor. Hypertension was recorded based on patients’ self-report or if patients were on hypertensive medication and/or had a repeatedly elevated BP reading; systolic BP > 140 mmHg or diastolic BP > 90 mmHg (measured at least twice).
Non-fasting blood samples were drawn to measure lipid and glucose profiles. Sex specific cut-off of 1.3 mmol/L was used to define low high-density lipoprotein (HDL) as per clinical practice guidelines [15]. Diabetes was recorded based on self-report (verified with patient held-record), or if they were on diabetes medication and/or had a random blood glucose concentration of equal to or more than 11.0 mmol/L.
Date of cancer diagnosis was obtained from the medical records. Tumour profiles that were extracted from the pathological records included histology (invasive ductal carcinoma, invasive lobular carcinoma, others), pathological tumour size at presentation (≤5 cm, >5 cm, unknown) and number of positive axillary lymph nodes (pN: 0, 1–3, 4–9, ≥10, unknown). Tumour grading was determined using the Scarff–Bloom–Richardson classification (grade 1, 2 or 3, unknown). Presence of distant metastasis at initial diagnosis was determined radiologically (yes, no, unknown). Cancer stage included pathological/radiological Tumour, Node, Metastasis (TNM) staging based on American Joint Committee on Cancer (AJCC) 7th edition (I, II, III or IV). Hormonal receptor status was determined via immunohistochemistry and deemed positive when >1% of cells stained positive (progesterone and oestrogen receptor (ER) expression (positive, negative, unknown)). For HER2 status, only tumours with an immunohistochemistry score of 3+ were regarded as HER2 overexpressed (positive), whereas those with score of 1+ were deemed negative. Expressions of 2+ were regarded as equivocal and underwent further in situ hybridisation testing for HER2 gene amplification.
Comorbidity measurement
In the current analysis, Charlson Comorbidity Index (CCI) was calculated for all patients using data on presence of comorbidities at baseline, which had been originally extracted from patients’ internal (hospital-based records) as well as external medical records (e.g. patient-held medical records, diabetes mini green book, discharge summaries) at the time of study recruitment, i.e. within 8 weeks of diagnosis [16]. While the original CCI is a weighted score that is based on both the number and severity of 19 pre-defined comorbid conditions including cancer, in the current study presence of cancer (weight = 1) or distant metastasis (weight = 6) was not scored. As a result, all patients were only scored if they had the following conditions: congestive heart failure (weight = 1), myocardial infarct (weight = 1), cerebrovascular disease (weight = 1), chronic pulmonary disease (weight = 1), paraplegia (weight = 2), dementia (weight = 1), diabetes without complications (weight = 1), diabetes with complications (weight = 2), mild liver disease (weight = 1), moderate or severe liver disease (weight = 3), peptic ulcer disease (weight = 1), peripheral vascular disease (weight = 1), rheumatologic disease (weight = 1), renal disease (weight = 2) and human immunodeficiency virus/acquired immune deficiency syndrome (weight = 6) [17].
Based on the presence of comorbidities, the corresponding weights were added to produce a cumulative score for every patient that we refer to as a modified CCI (ranging from 0 to 8). The score was stratified into quartiles with higher quartiles indicating higher comorbidity burden; Quartile 1 (score 0), Quartile 2 (score 1), Quartile 3 (score 3) and Quartile 4 (score ≥ 3).
In the current study, cardiometabolic comorbidities [18] included cardiovascular disease and diabetes mellitus [19] as well as their risk factors, namely hypertension, dyslipidaemia and obesity [20, 21].
Statistical analysis
Descriptive statistics were used to summarise patient characteristics. The demographic and pathological profiles were compared across the patients based on their burden of comorbidities using χ² test.
Multivariable logistic/linear regression analyses were used to determine the association between modified CCI quartiles and pathological risk factors. We also measured the association between specific comorbidities (hypertension, diabetes, obesity, low HDL level, pre-existing cardiovascular disease) and pathological characteristics.
Odds ratio (OR) with a 95% CI that does not include 1 was considered as statistically significant. Models were adjusted for age, ethnicity and type of hospital, as they may be associated with both comorbidity and pathological factors of breast cancer. Data were analysed using IBM Statistical Package for the Social Sciences version 26.
Results
In the present analysis, 41 (1.6%) patients with incomplete information on pathological/radiological cancer staging were excluded, leaving 2,501 patients with invasive cancers (stage I–IV). Median age at diagnosis was 53 years (25th percentile: 45 years, 75th percentile: 62 years). A majority of patients were Chinese (54.8%), followed by Malays (32.0%), Indians (11.4%) and other ethnicities (1.8%). Median tumour size at presentation was 2.5 cm (25th percentile: 2.0 cm, 75th percentile: 4.0 cm), whereby a fifth of patients presented with tumours measuring larger than 5 cm. Approximately half of the patients had lymph node involvement at initial diagnosis. Overall, 24% presented with stage I disease at initial diagnosis, followed by 39% with stage II, 28% with stage III and 10% with stage IV breast cancer. Close to a quarter (24%) of the cohort comprised women presenting with late cancer stages (comprising stage IIIb, IIIc and IV).
Very few patients were classified as having a comorbidity based on self-report alone ((hypertension: 15), (hypercholesterolaemia: 9)). However, given that HDL level was measured solely from blood samples, data were missing in 20.6%.
About 65% of women with breast cancer were found to have at least one comorbidity at initial diagnoses, of which cardio-metabolic comorbidities were most prevalent. Common comorbidities comprised dyslipidaemia (67.5%), hypertension (44.3%) and diabetes (17.3%). About 18% were obese. Other comorbidities included obstructive airway diseases (n = 47), thyroid diseases (n = 32), peptic ulcer disease (n = 27), chronic kidney disease (n = 19) and liver diseases (n = 16).
Median score of the modified CCI was 1.0 (25th percentile: 0 score, 75th percentile: 2 score). Patients aged 65 or above were more likely to be in the higher quartiles of CCI (Table 1). Significant ethnic variations were observed where Indian women with breast cancer were more likely to present with higher prevalence of comorbidities compared to their Chinese and Malay counterparts (Table 1). Among the Indian patients with breast cancer, hypertension (56.7%), and diabetes mellitus (39.1%) were the most common comorbidities. Among the Malays, hypertension (53.1%) and obesity (32.2%) were most prevalent, whereas in the Chinese, hypertension (36.1%) and diabetes mellitus (8.9%) largely prevailed. Patients from public hospitals appeared to have more comorbidities compared to their counterparts managed in private hospital.
A higher proportion of patients with higher modified CCI scores presented with larger tumours compared to their counterparts with lower modified CCI scores (p = 0.002). Patients with lower modified CCI scores were more likely to present with pathologically favourable features compared to patients in the higher quartiles of modified CCI scores, such as progesterone receptor (PR) positive status and HER2 negative status. However, no associations were observed with tumour histology, tumour grade or ER status (Table 2). We also observed a significant association between higher quartiles of modified CCI and tumour size in linear regression analysis after adjusting for age; CCI quartile 4 versus CCI quartile 1: B = 0.91 (95% CI: 0.24, 1.58), p = 0.008 (results not shown). Likewise, higher quartiles of modified CCI were associated with increasing number of positive lymph nodes after adjustment for age, CCI quartile 3 versus CCI quartile 1: B = 1.01 (95% CI: 0.02, 2.01), p = 0.046 (results not shown). These associations nonetheless attenuated following additional adjustment for ethnicity and type of hospital.
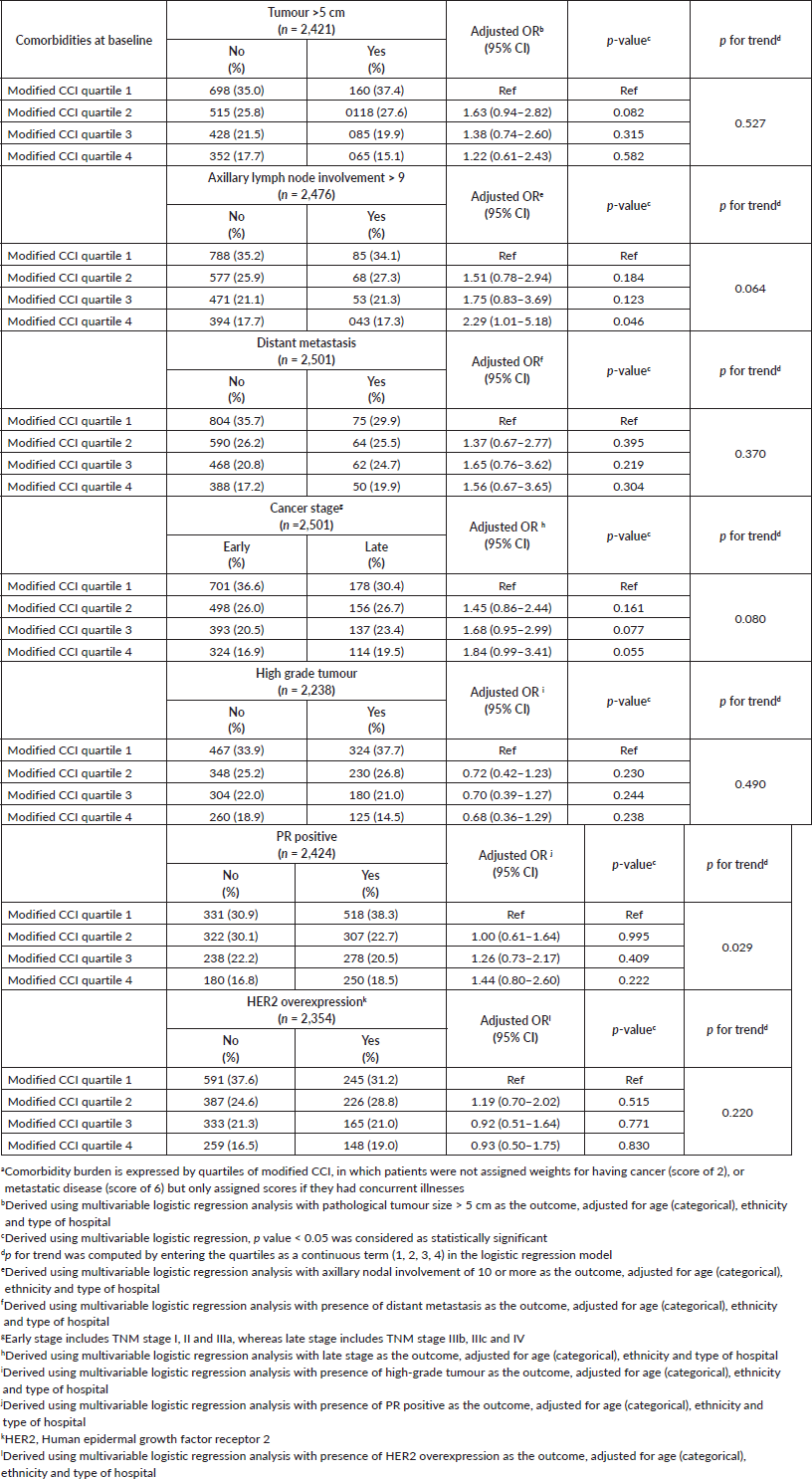
Following adjustment for age, ethnicity and type of hospital, multivariable logistic regression analyses showed that those in the higher quartiles of modified CCI were more likely to present with high lymph node burden (>9 positive nodes) compared to quartile 1, with a corresponding adjusted OR of 1.51 (95% CI: 0.78–2.94) for quartile 2, 1.75 (95% CI: 0.83–3.69) for quartile 3 and 2.29 (95% CI: 1.01–5.18) for quartile 4; p for linear trend test = 0.064. While those in quartile 4 of the modified CCI also tended to be associated with larger tumours, higher odds of distant metastasis and late stages compared to those in the lowest quartile, findings were not statistically significant (Table 3). Tumour grade and HER2 overexpression however were not independently associated with modified CCI.
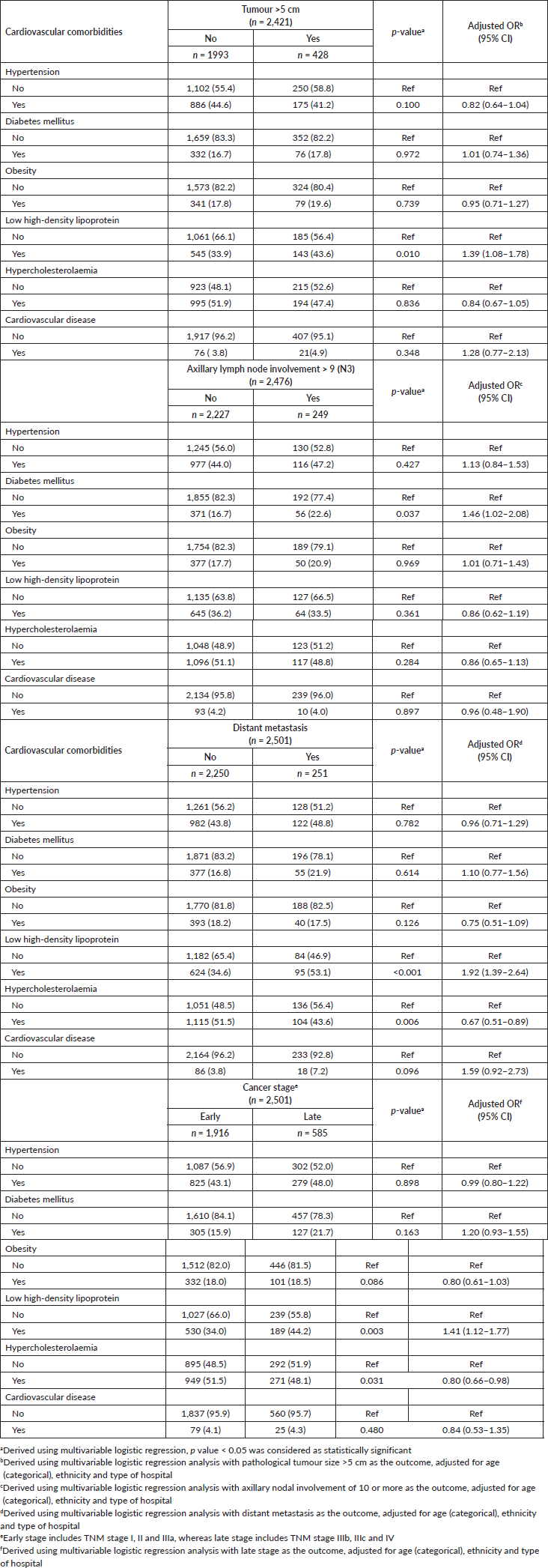
Several cardio-metabolic conditions were found to be specifically associated with unfavourable pathological profiles (Table 4). Low HDL levels, for instance, was associated with large tumours (>5 cm) (adjusted OR: 1.39, 95 CI%: 1.08–1.78), as well as distant metastasis (adjusted OR: 1.92, 95 CI%: 1.39–2.64), and hence late cancer stages at diagnoses (adjusted OR: 1.41, 95 CI%: 1.12–1.77). Likewise, hypercholesterolaemia was inversely associated with all the above factors. Diabetes mellitus was significantly associated with increased odds of higher nodal metastasis burden (pN3) at diagnosis (adjusted OR: 1.46, 95 CI%: 1.02–2.08). Although diabetes also appeared to be associated with distant metastasis, as well as late cancer stages at diagnoses, the findings were not statistically significant.
Post hoc analyses were also conducted where cardio-metabolic comorbidities, namely cardiovascular diseases, hypertension, diabetes, obesity and low HDL were grouped into three categories (0, 1, ≥2 comorbidities). Univariable logistic regression analysis showed that patients with no cardiometabolic comorbidities were significantly associated with favourable pathological features including smaller tumours (<2 cm), no nodal involvement and also absence of distance metastasis or HER2 overexpression, compared to their counterparts with two or more (clustering) cardiometabolic comorbidities (results not shown). Multivariable analyses revealed that compared to breast cancer patients with neither cardiovascular diseases, hypertension, diabetes, low HDL nor obesity at baseline, women who presented with clustering of these comorbid conditions were more likely to be associated with larger tumours, distant metastasis and late cancer stages (Table 5).
Sensitivity analyses adjusting for treatment with antihypertensives, statin and antidiabetic drugs in the multivariable analyses examining the association of hypertension, hypercholesterolaemia/low HDL and diabetes with distant metastasis/cancer stage, respectively, did not materially change the study inferences.
Table 1. Baseline characteristics of women newly diagnosed with breast cancer by comorbidity burdena.
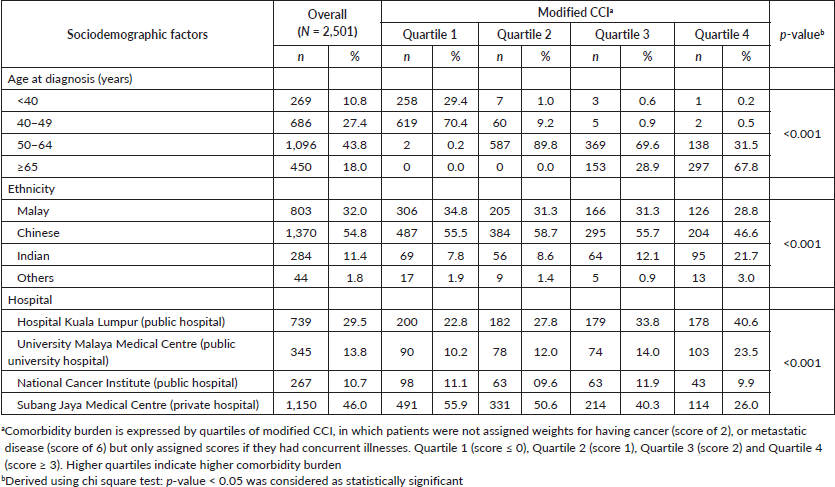
Table 2. Clinicopathological features of breast cancer by comorbidity burden in 2,501 women.
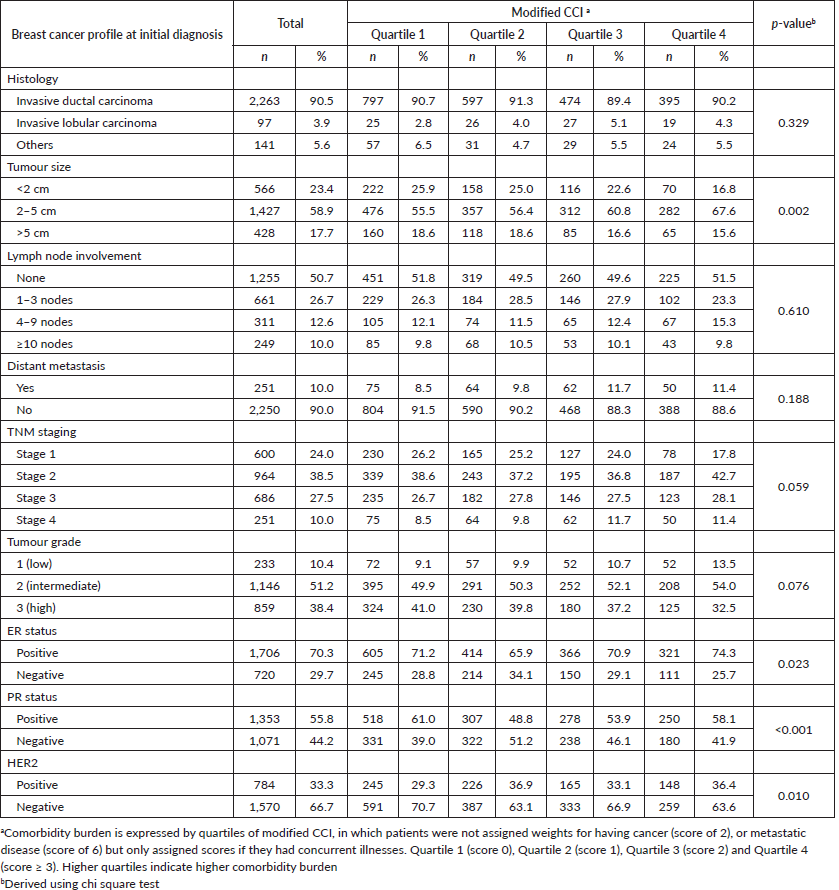
Table 3. Association between baseline comorbidity burdena and pathological profile of breast cancer.
Discussion
In this relatively young cohort of women who were newly diagnosed with breast cancer, close to two-thirds were found to have at least one comorbidity at initial presentation. Apart from age, ethnicity was also significantly associated with baseline burden of comorbidities. The presence of cardio-metabolic comorbidities seemed to be independently associated with unfavourable pathological features at initial breast cancer diagnosis including larger tumours, higher lymph node involvement and distant metastasis. Specifically, low levels of HDL were significantly associated with larger tumours, distant metastasis and late cancer stages, while presence of diabetes mellitus at baseline was significantly associated with higher nodal metastasis burden.
Table 4. Association between baseline cardio-metabolic comorbidities and the tumour profile at initial diagnosis with breast cancer.
Our findings of higher prevalence of cardiometabolic comorbidities among women with breast cancer who are of Indian ethnicity tally with the reports from the recent National Health and Morbidity Survey, which is a nationwide study of the general Malaysian population [22]. The ethnic differences, apart from being attributed to variations in dietary preferences and lifestyle behaviours [23], may also be explained by genetic factors. For instance, genetic variants that are associated with increased risks of cardiovascular diseases have been found to be more prevalent among people of Indian origin [24]. It is thought that all of the above may also apply to Indian women with breast cancer.
While the association between comorbidity and cancer stage at diagnosis is well-established, the direction and magnitude of the association appear to vary [3]. Comorbidities may influence tumour biology through direct and indirect mechanisms, including manifestation of local and systemic inflammation, and alterations to tumour microenvironment [9]. Conceivably, these pathophysiological events may in turn influence carcinogenesis and progression of cancer including its propensity to metastasise. This is well supported by our present findings where positive associations were observed between higher comorbidity burden and more aggressive pathological features including, higher nodal burden and presence of distant metastasis. The associations were less apparent when all comorbidities were included for distant metastasis comparing quartile 4 of modified CCI versus quartile 1, indicating that there may be specific biological mechanisms that apply only for a specific subset of comorbidities. This is further supported by our post hoc analyses including only the cardiovascular diseases, hypertension, diabetes, obesity and low HDL, where we have demonstrated significantly stronger findings for distant metastasis comparing those with ≥2 cardiometabolic diseases versus no cardiometabolic disease. While it is acknowledged that certain medications to treat common comorbidities, such as statins and anti-inflammatory agents may confer a protective effect against cancer progression [25–27], sensitivity analyses adjusting for use of such medications did not appear to alter study inferences.
Table 5. Association between clustering of cardio-metabolic comorbidities at baseline and the tumour profile at initial diagnosis with breast cancer.
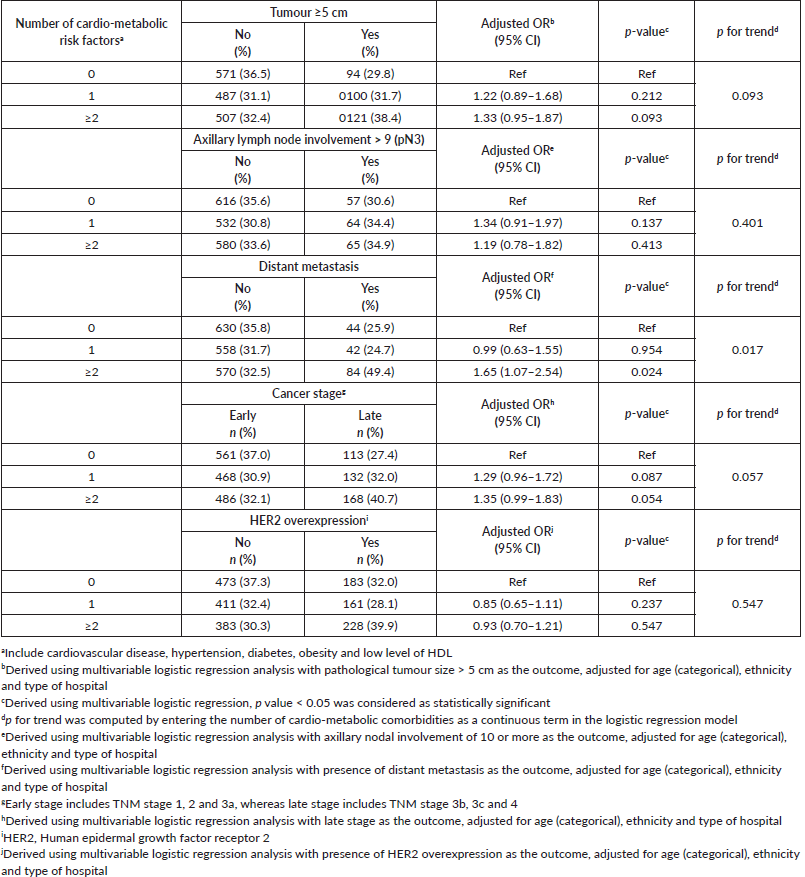
The significant association between low HDL and risk of distant metastasis that we have observed appears to be in line with prior literature, namely from large clinical trials where an inverse association between HDL levels and risk of breast cancer has been reported [11]. In a study comparing women with breast cancer and healthy controls, significantly lower levels of HDL and elevated levels of total cholesterol were reported in those with breast cancer [28]. It has been posited that lower levels of HDL influence carcinogenesis via cell entry cycle regulation, cytokine production and antioxidation [29]. In vitro studies have also reported that under oxidative stress, oxidatively modified HDL can promote cell proliferation, migration, invasion and adhesion, all of which may contribute to tumour progression [11].
Multiple mechanisms have been proposed in explaining how diabetes could influence cancer progression, including hyperinsulinaemia, hyperglycaemia and chronic inflammation [10]. The activation of high circulating levels of insulin and insulin-like growth factors among people with diabetes, both of which promote cell proliferation and affect cell apoptosis, has been linked with a higher risk of incident cancer, as well as cancer metastasis and recurrence [10]. Furthermore, it has been shown that the composition and structure of HDL are often altered in patients with diabetes, leading to increased proinflammatory actions [11].
While it appeared that cancer stage at diagnosis was not significantly different between the various quartiles of CCI, more meaningful differences were observed when analyses were confined to cardio-metabolic comorbidities. Moreover, associations were even more apparent when specific comorbidities were examined in relation to specific tumour characteristics (tumour size, nodal involvement, distant metastasis). It must be further noted that certain conditions that can only be measured via laboratory tests were also found to be associated with aggressive tumour features. Intuitively, the bigger the tumour, the easier it should be for women to self-detect it. However, as we did not have data on breast cancer screening behaviour, we were unable to determine the relative role and magnitude of biological and non-biological factors in influencing cancer stage at diagnosis. While it is often not straightforward to disentangle the interplay between biological and non-biological factors in influencing cancer stage at diagnosis, our present findings at the least challenge the notion that the association between cardio-metabolic comorbidities and advanced cancer stages at diagnoses is explained purely by delays in cancer diagnosis.
Strengths and limitations
To the best of authors’ knowledge, this is the first study that consecutively measured the baseline prevalence of common comorbidities in women who were newly diagnosed with breast cancer, which were then examined in relation to their clinicopathological characteristics at initial diagnosis. Nonetheless, it is acknowledged that there are intersections between many comorbid conditions, and it is plausible that some of the associations are bidirectional, rendering it difficult to disentangle their effects. Furthermore, the CCI is limited in terms of the comorbid conditions that it encompasses. Despite its shortcoming, the CCI is still commonly used due to a lack of a gold standard for measuring comorbidities in cancer populations [30]. Last but not least, it remains possible that we may have missed some comorbidities, particularly early disease or less severe conditions which may have been undiagnosed.
Conclusion
Taken together, our study findings seem to point towards the presence of underlying biological mechanisms linking cardiometabolic comorbidities and pathological profiles of breast cancer among women who are newly diagnosed. This in turn implies that the late stage at cancer diagnosis that is observed in women with breast cancer may not only be attributed to delay in timeliness of diagnoses arising from patient, physician and health systems-related factors [4], but also by pathophysiological interactions between common comorbidities such as dyslipidaemia as well as diabetes, and tumour biology. Given the poorer survival among breast cancer patients with comorbidities, there is a need for more evidence to understand the interactions, magnitude and impact of both socio-structural and biological factors that lead to more aggressive cancers at diagnosis [1, 2]. From a healthcare system’s point of view, there needs to be a paradigm shift from focusing on cancer patients with a narrow lens (single disease approach) to a wider lens that encompasses the management of multimorbidities. A coordinated care model therefore will lead to timely diagnosis and optimal management of morbidities, which in turn will result in improved clinical and patient-centred outcomes, as well as a reduction in healthcare costs for both patients and healthcare systems [31, 32].
Acknowledgments
The authors would like to thank the Director-General of Health, Ministry of Health Malaysia for his permission to publish this study.
Authors’ contributions
NB: Concept and design, statistical analysis, interpretation of data, drafting of manuscript, critical revision of manuscript and final approval. SS: Data acquisition, statistical analysis, interpretation of data, drafting of manuscript, critical revision of manuscript and final approval. YCK: Data acquisition, drafting of manuscript, critical revision of manuscript and final approval. CHY: Data acquisition, interpretation of data, critical revision of manuscript and final approval. MT: Critical revision of manuscript and final approval. PJ: Critical revision of manuscript and final approval. HZ: Data acquisition, critical revision of manuscript and final approval. NAT: Data acquisition, critical revision of manuscript and final approval. MHS: Data acquisition, critical revision of manuscript and final approval. DS: Statistical analysis, interpretation of data, drafting of manuscript, critical revision of manuscript and final approval.
Conflicts of interest
The authors have no conflicts of interest to report.
Funding
The authors have no funding to declare for this work.
Ethics approval
Ethical approval was approved by the Medical Research and Ethics Committee (NMRR-15-803-24756) and University of Malaya Research Ethics Committee (UMMEC 896.150). Written informed consent was obtained from all participants. Ethical principles according to Helsinki’s declaration were observed throughout the conduct of the study.
References
1. Braithwaite D, Moore DH, and Satariano WA, et al (2012) Prognostic impact of comorbidity among long-term breast cancer survivors: results from the LACE study Cancer Epidemiol Biomarkers Prev 21(7) 1115–1125 https://aacrjournals.org/cebp/article/21/7/1115/69300/Prognostic-Impact-of-Comorbidity-among-Long-Term https://doi.org/10.1158/1055-9965.EPI-11-1228 PMID: 22573797 PMCID: 3470873
2. Land LH, Dalton SO, and Jørgensen TL, et al (2012) Comorbidity and survival after early breast cancer. A review Crit Rev Oncol Hematol 81(2) 196–205 https://www.sciencedirect.com/science/article/pii/S1040842811000680?via%3Dihub https://doi.org/10.1016/j.critrevonc.2011.03.001
3. Sarfati D, Koczwara B, and Jackson C (2016) The impact of comorbidity on cancer and its treatment CA Cancer J Clin 66(4) 37–50 https://doi.org/10.3322/caac.21342
4. Lee L, Cheung WY, and Atkinson E, et al (2011) Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review J Clin Oncol 29(1) 106–101 https://doi.org/10.1200/JCO.2010.31.3049
5. Renzi C, Kaushal A, and Emery J, et al (2019) Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms Nat Rev Clin Oncol 16(12) 746–761 https://doi.org/10.1038/s41571-019-0249-6 PMID: 31350467
6. Gurney J, Sarfati D, and Stanley J (2015) The impact of patient comorbidity on cancer stage at diagnosis Br J Cancer 113(9) 1375–1380 https://doi.org/10.1038/bjc.2015.355 ">https://doi.org/10.1038/bjc.2015.355 PMID: 26461060 PMCID: 4815795
7. Vaeth PA, Satariano WA, and Ragland DR (2000) Limiting comorbid conditions and breast cancer stage at diagnosis J Gerontol A Biol Sci Med Sci 55(10) M593–M600 https://academic.oup.com/biomedgerontology/article/55/10/M593/636250?login=false https://doi.org/10.1093/gerona/55.10.M593 PMID: 11034232
8. Fleming ST, Pursley HG, and Newman B, et al (2005) Comorbidity as a predictor of stage of illness for patients with breast cancer Med Care 43(2) 132–140 https://journals.lww.com/lwwmedicalcare/Abstract/2005/02000/Comorbidity_as_a_Predictor_of_Stage_of_Illness_for.6.aspx https://doi.org/10.1097/00005650-200502000-00006 PMID: 15655426
9. Panigrahi G and Ambs S (2021) How comorbidities shape cancer biology and survival Trends Cancer 7(6) 488–495 https://www.sciencedirect.com/science/article/abs/pii/S240580332030337X?via%3Dihub https://doi.org/10.1016/j.trecan.2020.12.010 PMID: 33446449 PMCID: 8137526
10. Giovannucci E, Harlan DM, and Archer MC, et al (2010) Diabetes and cancer: a consensus report Diabetes Care 33(7) 1674–1685 https://diabetesjournals.org/care/article/33/7/1674/39273/Diabetes-and-CancerA-consensus-report https://doi.org/10.2337/dc10-0666 PMID: 20587728 PMCID: 2890380
11. Cedó L, Reddy ST, and Mato E, et al (2019) HDL and LDL: potential new players in breast cancer development J Clin Med 8(6) 853 https://doi.org/10.3390/jcm8060853 PMID: 31208017 PMCID: 6616617
12. Johnson CB, Davis MK, and Law A, et al (2016) Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients Can J Cadiol 32(7) 900–907 https://www.sciencedirect.com/science/article/pii/S0828282X16300526?via%3Dihub https://doi.org/10.1016/j.cjca.2016.04.008
13. Subramaniam S, Kong YC, and Zaharah H, et al (2021) Baseline cardiovascular comorbidities, and the influence on cancer treatment decision-making in women with breast cancer Ecancermedicalscience 21(15) 1293 https://ecancer.org/en/journal/article/1293-baseline-cardiovascular-comorbidities-and-the-influence-on-cancer-treatment-decision-making-in-women-with-breast-cancer
14. Su TT, Amiri M, and Mohd Hairi F, et al (2015) Prediction of cardiovascular disease risk among low-income urban dwellers in metropolitan Kuala Lumpur, Malaysia Biomed Res Int 2015 516984 https://www.hindawi.com/journals/bmri/2015/516984/ PMID: 25821810 PMCID: 4363497
15. Ministry of Health Malaysia (2017) Clinical Practice Guidelines. Management of Dyslipidaemia 5th edn http://www.moh.gov.my/moh/resources/Penerbitan/CPG/Cardiovascular/4.pdf
16. Ramli AS, Selvarajah S, and Daud MH, et al (2016) Effectiveness of the EMPOWER-PAR intervention in improving clinical outcomes of type 2 diabetes mellitus in primary care: a pragmatic cluster randomised controlled trial BMC Fam Pract 17(1) 157 https://doi.org/10.1186/s12875-016-0557-1 PMID: 27842495 PMCID: 5109682
17. Charlson ME, Pompei P, and Ales KL, et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation J Chronic Dis 40(5) 373–383 https://www.sciencedirect.com/science/article/pii/0021968187901718?via%3Dihub https://doi.org/10.1016/0021-9681(87)90171-8 PMID: 3558716
18. Zhang D, Tang X, and Shen P, et al (2019) Multimorbidity of cardiometabolic diseases: prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study BMJ Open 9(3) e024476 https://bmjopen.bmj.com/content/9/3/e024476.long https://doi.org/10.1136/bmjopen-2018-024476 PMID: 30833320 PMCID: 6443196
19. de Waard AM, Hollander M, and Korevaar JC, et al (2019) Selective prevention of cardiometabolic diseases: activities and attitudes of general practitioners across Europe Eur J Public Health 29(1) 88–93 https://academic.oup.com/eurpub/article/29/1/88/5054640 https://doi.org/10.1093/eurpub/cky112 PMCID: 6345147
20. Baygi F, Herttua K, and Jensen OC, et al (2020) Global prevalence of cardiometabolic risk factors in the military population: a systematic review and meta-analysis BMC Endocr Disord 20(1) 8 [https://bmcendocrdisord.biomedcentral.com/articles/10.1186/s12902-020-0489-6] https://doi.org/10.1186/s12902-020-0489-6 PMID: 31931788 PMCID: 6958577
21. Zullig LL, Sung AD, and Khouri MG, et al (2022) Cardiometabolic comorbidities in cancer survivors: JACC: CardioOncology state-of-the-art review JACC CardioOncol 4(2) 149–165 https://www.sciencedirect.com/science/article/pii/S2666087322002198?via%3Dihub https://doi.org/10.1016/j.jaccao.2022.03.005 PMID: 35818559 PMCID: 9270612
22. Institute for Public Health (IPH), National Institutes of Health, and Ministry of Health Malaysia (2019) National Health and Morbidity Survey (NHMS) 2019: Vol. I: NCDs – Non-Communicable Diseases: Risk Factors and Other Health Problems https://iku.moh.gov.my/images/IKU/Document/REPORT/NHMS2019/Report_NHMS2019-NCD_v2.pdf
23. Tan AK, Dunn RA, and Yen ST (2011) Ethnic disparities in metabolic syndrome in Malaysia: an analysis by risk factors Metab Syndr Relat Disord 9(6) 441–451 https://doi.org/10.1089/met.2011.0031 PMID: 21815810 PMCID: 3225058
24. Mahadevan L, Yesudas A, and Sajesh PK, et al (2014) Prevalence of genetic variants associated with cardiovascular disease risk and drug response in the Southern Indian population of Kerala Indian J of Hum Genet 20(2) 175–184
25. Bardia A, Olson JE, and Vachon CM, et al (2011) Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study Breast Cancer Res Treat 126(1) 149–155 https://doi.org/10.1007/s10549-010-1074-x
26. Sassano A and Platanias LC (2008) Statins in tumor suppression Cancer Lett 260(1–2) 11–19 https://www.sciencedirect.com/science/article/pii/S0304383507005733?via%3Dihub https://doi.org/10.1016/j.canlet.2007.11.036 PMID: 18180097
27. Takkouche B, Regueira-Méndez C, and Etminan M (2008) Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis J Natl Cancer Inst 100(20) 1439–1447 https://academic.oup.com/jnci/article/100/20/1439/899554?login=false https://doi.org/10.1093/jnci/djn324 PMID: 18840819
28. Michalaki V, Koutroulis G, and Syrigos K, et al (2005) Evaluation of serum lipids and high-density lipoprotein subfrac-tions (HDL2, HDL3) in postmenopausal patients with breastcancer Mol Cell Biochem 268(1–2) 19–24 https://link.springer.com/article/10.1007/s11010-005-2993-4 https://doi.org/10.1007/s11010-005-2993-4 PMID: 15724433
29. Saba AB and Ajibade T (2012) Role of Lipoproteins in Carcinogenesis and in Chemoprevention (IntechOpen) pp 647–622 https://www.intechopen.com/chapters/39530
30. Sarfati D (2012) Review of methods used to measure comorbidity in cancer populations: no gold standard exists J Clin Epidemiol 65(9) 924–933 https://www.sciencedirect.com/science/article/pii/S0895435612000789?via%3Dihub https://doi.org/10.1016/j.jclinepi.2012.02.017 PMID: 22739245
31. Powers BW, Modarai F, and Palakodeti S, et al (2020) Impact of complex care management on spending and utilization for high-need, high-cost Medicaid patients Am J Manag Care 26(2) E57–E63 https://www.ajmc.com/view/impact-of-complex-care-management-on-spending-and-utilization-for-highneed-highcost-medicaid-patients https://doi.org/10.37765/ajmc.2020.42402 PMID: 32059101
32. Hershey DS and Given BA (2020) Improving care coordination for comorbidity and Cancer: a necessity for patients with cancer Cancer Nurs 43(1) 86–87 https://journals.lww.com/cancernursingonline/Fulltext/2020/01000/Improving_Care_Coordination_for_Comorbidity_and.11.aspx https://doi.org/10.1097/NCC.0000000000000780






