Neoadjuvant chemotherapy in technically unresectable head and neck cancers: a retrospective audit
Bal Krishna Mishra1, Akhil Kapoor1, Anuj Gupta1, Bipinesh Sansar1, Arpita Singh1, Somnath Roy1, Tanmoy Mandal1, Sujay Srinivas1, Sudeep Das1, Aseem Mishra2, Ashutosh Mukherjee3, Sambit Nanda3 and Kurupathy Sambasivaiah1
1Department of Medical Oncology, Mahamana Pandit Madan Mohan Malviya Cancer Centre & Homi Bhabha Cancer Hospital, Tata Memorial Centre, Varanasi, 221005, India
2Department of Head and Neck Surgery, Mahamana Pandit Madan Mohan Malviya Cancer Centre & Homi Bhabha Cancer Hospital, Tata Memorial Centre, Varanasi, 221005, India
3Department of Radiation Oncology, Mahamana Pandit Madan Mohan Malviya Cancer Centre & Homi Bhabha Cancer Hospital, Tata Memorial Centre, Varanasi, 221005, India
Abstract
Background: The data regarding the use of neoadjuvant chemotherapy in technically unresectable head and neck cancer (HNC) is limited and real-world studies are needed to look for the efficacy and toxicities of this approach.
Patients and methods: This is a retrospective study conducted in the Medical Oncology department of our hospital. All technically unresectable HNC patients who underwent neoadjuvant chemotherapy between May 2018 and May 2020 were included in this analysis. Patients received three-drug regimen docetaxel, cisplatin and 5-fluorouracil (DCF) regimen, two-drug regimens included docetaxel + cisplatin, paclitaxel + carboplatin both weekly and 3-weekly. The resectability assessment was done clinically and radiologically after completing three neoadjuvant cycles. Overall survival was calculated from the first day of chemotherapy to the date of last follow-up or date of death.
Results: A total of 119 patients received neoadjuvant chemotherapy during the specified time. Response assessment showed partial response in 41.9% of patients with three-drug regimens and 37.5% of patients with other regimens. Out of 119 patients, 56 (47%) patients were offered radical intent therapy. Resectability was achieved in 32.3% of three-drug regimen patients and 26.1% of other patients. Surgery was feasible in 33 (27.7%) patients, and postoperative radiotherapy and concurrent chemotherapy were done in 30 patients (25.2%), and surgery with only postoperative radiotherapy was done in 3 patients (2.5%). Radical chemoradiotherapy was done in 23 patients (19.3%). The estimated median survival for patients who could undergo surgery was 18 months [95% confidence interval (CI), 14.9-21.0], and nonsurgical patients were 9 months (95% CI, 7.3–10.6) (p = 0.0001).
Conclusion: Our study shows that neoadjuvant chemotherapy in technically unresectable HNC patients can make the disease resectable in around one-third of the patients. The patients who could undergo surgery after neoadjuvant chemotherapy had significantly improved survival as compared to those who could not.
Keywords: neoadjuvant chemotherapy, technically unresectable, head and neck cancers
Correspondence to: Akhil Kapoor
Email: kapoorakhil1987@gmail.com
Published: 02/11/2022
Received: 11/07/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Head and neck cancer (HNC) is a significant health problem in India due to tobacco chewing, especially in the male population [1]. Another reality is their presentation as a locally advanced disease which ranges from 58.3% to 76.9% for various subsites in various parts of countries [2].
Surgery is the primary treatment for oral cavity cancer [3–8]. Advanced stage HNC is mainly treated by combined modality treatment, in which surgery followed by radiotherapy with concurrent chemotherapy is delivered. However, in oral cavity cancers, if surgery is not technically feasible, the treatment is essentially palliative. Concurrent chemoradiation has been tried in these patients; however, the outcomes are dismal with a median progression-free survival (PFS) of 6.4 months. Besides, it is challenging to get a clear margin in locally advanced HNC. The local recurrence rate is higher in patients with close or positive margins than negative margin patients [9]. Thus, it is necessary to get surgical resection with negative margins [10]. Decisions regarding resectability and intent of the treatment in borderline cases should always be made by a multidisciplinary team. There is already some data to suggest that a proportion of carefully selected patients with borderline unresectable tumours who would not be offered surgery might be made resectable using induction chemotherapy. TAX 323 and 324 are regarded as landmark studies that have used neoadjuvant chemotherapy in locally advanced HNC. The response rates with the three-drug regime were to the tune of 68%; however oral cavity cancers constituted a minority of the treated patient population in these studies. The data regarding the same is conflicting and real-world studies are needed to look for the efficacy and toxicities of this approach.
Materials and methods
General study details
This is a retrospective study conducted in the Medical Oncology department of our hospital. All technically unresectable HNC patients who underwent neoadjuvant chemotherapy (NACT) between May 2018 and May 2020 were included in this analysis. Since this is an audit of the standard institutional practice, ethical approval was not sought. The study was conducted according to ethical guidelines established by the Declaration of Helsinki and other guidelines like Good Clinical Practice Guidelines and those established by the Indian Council of Medical Research.
All HNC patients were evaluated by a multidisciplinary clinic, including surgical oncologists, radiation oncologists, medical oncologists and radiologists. All technically unresectable patients were planned for neoadjuvant chemotherapy treatment. The reasons for labeling the disease as technically unresectable were as follows:
1. Disease reaching up to zygoma and/or soft tissue swelling up to zygoma
2. Infratemporal fossa involvement
3. Extensive soft tissue involvement reaching up to the hyoid cartilage and extensive skin infiltration
4. Fix lymph nodes with vascular involvement.
All technically unresectable patients received neoadjuvant chemotherapy with various regimens, which includes a three-drug regimen DCF (docetaxel + cisplatin + 5-fluorouracil), two-drug regimens include DC (docetaxel + cisplatin), PC (paclitaxel + carboplatin) both weekly and 3- weekly. The number of cycles given was three in 3-weekly regimens and eight in weekly PC regimens. Docetaxel was administered at dose of 75 mg/m2 over 1 hour on day 1, cisplatin at 75 mg/m2 over 1 hour divided in 2 days, 5-fluorouracil at dose of 750 mg/m2 continuous infusion over 24 hours day 1–5 via a peripherally inserted central catheter. Paclitaxel was given at dose of 175 mg/m2 on day 1 and carboplatin at Area under curve (AUC) 5 for 3-weekly protocol, while the dose was 80 mg/m2 and AUC2, respectively, for weekly protocol. Patients having grade ¾ toxicities were considered for next cycle of chemotherapy at 80% doses.
Toxicity data were collected mainly for anaemia, febrile neutropenia, thrombocytopenia, vomiting, diarrhoea and cardiac toxicity to see the feasibility of all regimens as per Common Terminology Criteria for Adverse Events (CTCAE) version 4.02. All standard DCF regimen patients received prophylactic G-CSF. All patients treated with other regimens also received granulocyte colony stimulating factor (G-CSF). if they developed febrile neutropenia in the previous cycle.
The resectability assessment was done clinically and radiologically after completing three neoadjuvant cycles. All patients were assessed for resectability in the multidisciplinary joint clinic. All patients who were not found resectable were planned for radical chemoradiation (CT RT), radical radiotherapy, palliative radiotherapy, palliative chemotherapy and best supportive care as per joint clinic decision.
We looked at age, sex, site, histopathology, the indication of neoadjuvant chemotherapy, number of chemotherapy cycles, type of chemotherapy regimen, type of treatment given after completion of neoadjuvant therapy, response and survival for all patients.
Statistics
We did statistical analysis by using Statistical Package for Social Sciences (SPSS) software version 16. We calculated overall survival (OS) from the first day of chemotherapy to the date of last follow-up or date of death.
Results
Baseline parameters
A total of 119 patients received neoadjuvant chemotherapy during the specified time period. Median age, male/female ratio, site, reason of neoadjuvant chemotherapy and type of chemotherapy regimen are reported in Table 1. 26.2% (n = 31) of patients received three drug-based regimens in comparison to 73.8% (n = 88) of patients who received two-drug regimen. In the three-drug regimen, standard DCF regimen was used. Two-drug regimens include DC, PC both weekly and 3-weekly. The cycles given were three in 3-weekly regimens and eight in weekly PC regimens. Out of 88 patients receiving doublet chemotherapy regimen, 42 (47.7%) received DC protocol, 30 (34.1%) received 3-weekly PC and 16 (18.2%) received weekly PC regimen.
Table 1. Baseline parameters.

Efficacy
Response assessment showed partial response (PR) in 41.9% of patients with three-drug regimens and 37.5% of patients with other regimens (Table 2). Complete response (CR) was not seen with any regimen. Resectability was achieved in 32.3% of three-drug regimen patients and 26.1% of other patients. Table 3 shows the responses as per the indication of NACT. Among all the indications of NACT, partial responses were highest for disease/oedema up to zygoma.
Toxicity
Grade 3 and 4 toxicities were reported with all types of regimens, and the main toxicities seen were anaemia, febrile neutropenia, thrombocytopenia, vomiting, diarrhoea and cardiac (Table 2). No death was reported because of toxicity, and except for anaemia which was more common in two-drug arm, none of the toxicity showed a significant difference in both arms.
Post-neoadjuvant therapy treatment
Out of 119 patients, 56 (47%) patients were offered radical intent therapy (Table 4). Surgery was feasible in 33 (27.7%) patients, and postoperative radiotherapy and concurrent chemotherapy were done in 30 patients (25.2%), and surgery with only postoperative radiotherapy was done in 3 patients (2.5%). Radical chemoradiotherapy was done in 23 patients (19.3%). Sixty three patients (53%) were not fit for any radical treatment, and 48 patients (40.4%) received palliative chemotherapy, 8 patients (6.7%) received palliative radiotherapy and 7 patients (5.9%) received only best supportive care. The dose of radiation used was 66–70 Gy/6–7 weeks with a fraction size of 2 Gy for radical radiotherapy and 60 Gy/6 weeks for postoperative radiotherapy with a fraction size of 2 Gy.
Overall survival
The median duration of follow-up was 27 months (95% CI, 15.1–38.9). Total 85 events had taken place during this time. The estimated median OS for the whole population is 12 months (95% CI, 10.1–13.9). The estimated median survival for surgical patients was 18 months (95% CI, 14.9–21.0) and nonsurgical patients were 9 months (95% CI, 7.3–10.6), and this was statistically significant (p = 0.0001) (Figure 1).
Discussion
Oral cavity cancers have a high prevalence in South-Central Asian men, with most of them presenting as locoregionally advanced disease. The advanced stage at presentation always remains a challenge to plan curative-intent treatment. Surgery for local and regional disease combined with adjuvant therapy, even in cases of advanced disease, can offer a very reasonable survival rate [18]. Various neoadjuvant chemotherapy trials in HNCs did not show any benefits [20, 21]. Patil et al [16] reported the data of 123 patients and demonstrated the effectiveness of induction chemotherapy in down-staging of tumours. He reported median OS for the whole population of 12.7 months, and at the same time, estimated median survival was not reached for patients who had undergone post-chemotherapy resection. The difference was statistically significant (p = 0.0001). In another study by Patil et al [19], 721 technically unresectable patients who received neoadjuvant chemotherapy were retrospectively analysed. 43% of patients able to undergo surgery and median OS was 19.6 months who received surgical treatment compared to 8.16 months in those patients in which surgery was not possible, contrary to other neoadjuvant chemotherapy trials. It was observed that the proportion of oral cavity cancers in all major trials conducted primarily in North America and Europe was around 14%–18%, and maybe this low percentage may be the reason for different results.
The issue of borderline resectability is much more frequent in oral cancer, and it may need a different approach for management. We offered neoadjuvant chemotherapy to only those technically unresectable patients having features mentioned in the method section.
NACT protocol used in our study was based on published literature documenting the efficacy of both three drugs, two drugs and other regimens [22, 23]. In our analysis, 26.2% of patients received three-drug regimens, and 73.8% received two-drug regimens or other regimens and were found to be efficacious. The PR rates were 41.9% with three-drug regimen and 37.5% with two-drug regimens, which are actually lower than those reported in other studies [24, 25]. The low response may be due to more advanced stage patients and more oral cavity patients in our cohort, which is traditionally associated with poor response [22, 25].
32.3% of patients in the three-drug arm versus 26% in another arm could undergo surgery in our study with R0 resection, which was a significant achievement. Median OS was 18 months in patients who had surgical resection with adjuvant chemoradiation or adjuvant radiation compared to 9 months who could not go for surgical resection and were treated with radical chemoradiation, radical chemoradiation, radical radiation and other therapies (p = 0.0001). It shows the positive impact of surgery on survival. This was in concordance with EORTC postoperative adjuvant chemoradiation trial; operable Stage IV oral cancers benefited from adjuvant chemoradiation [26, 27].
The median survival seen in our study is lower when compared to that reported for two-drug and three-drug regimens in the TAX 323 and TAX 324. This difference may be due to fewer oral cavity patients in the above trials [11, 12]. Also, the median follow-up is shorter as compared to TAX 323 and TAX 324. This can be another reason for a lower median survival. Multiple evidence suggests that patients who undergo surgery have a better outcome than those who did not undergo resection in oral cavity cancer [13–15]. Neoadjuvant chemotherapy followed by surgery may improve the overall outcome and lead to an increase in OS. We also observed in our study that a significant number of patients tolerated radical intent nonsurgical therapy, which may be CT RT or RT alone. The outcomes of these patients are also not inferior.
Table 2. Response rates achieved, resectability at the end of three cycles and toxicity analysis.

Table 3. Response as per the indication of NACT.

Table 4. Post-neoadjuvant treatment offered.
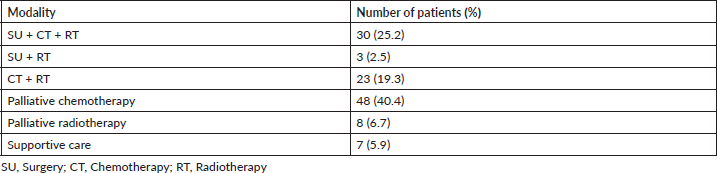

Figure 1. Consort diagram of all patients. CR, Complete response; PR, Partial response; SD, Stable disease; PD, Progressive disease; Sx, Surgery; CTRT, Chemoradiation; Pall RT, Palliative radiation; Pall CT, Palliative chemotherapy; BSC, Best supportive care.

Figure 2. Survival curve of resected patients versus other patients.
Conclusion
Our study shows that neoadjuvant chemotherapy in technically unresectable HNC patients can make the disease resectable in around one-third of the patients. The patients who could undergo surgery after neoadjuvant chemotherapy had significantly improved survival as compared to those who could not. The selection of these patients should be done by a multi-disciplinary team and all efforts should be done to make disease resectable to achieve optimal outcomes.
Conflicts of interest
Nil.
Funding
The authors did not receive any funding for conducting this study.
Availability of data and material
The raw data can be made available on appropriate request, while ensuring the anonymity of the study participants.
Authors’ contributions
The study conception and design was done by BK Mishra. Data collection was performed by BK Mishra, Akhil Kapoor, Anuj Gupta, Bipinesh Sansar, Arpita Singh, Somnath Roy, Tanmoy Mandal, Sujay Srinivas and Sudeep Das. Data analysis was performed by Akhil Kapoor and BK Mishra. The first draft of the manuscript was written by BK Mishra and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript and guarantee integrity of the entire study.
References
1. Report of National Cancer Registry Programme (2012-2016), National Centre for Disease Informatics and Research, National Cancer Registry Programme, Indian Council of Medical Research [https://ncdirindia.org/All_Reports/Report_2020/resources/AInitialPages.pdf] Data accessed: 22/04/22
2. Mathur P, Sathishkumar K, and Chaturvedi M, et al (2020) Cancer statistics, 2020: report from national cancer registry programme, India JCO Glob Oncol 6 1063–1075 https://doi.org/10.1200/GO.20.00122 PMID: 32673076 PMCID: 7392737
3. Shah JP and Gil Z (2009) Current concepts in management of oral cancer surgery Oral Oncol 45 394–401 https://doi.org/10.1016/j.oraloncology.2008.05.017
4. Scully C and Bagan JV (2008) Recent advances in oral oncology 2007: imaging, treatment and treatment outcomes Oral Oncol 44 211–215 https://doi.org/10.1016/j.oraloncology.2008.01.006 PMID: 18261952
5. Scully C and Bagan JV (2009) Oral squamous cell carcinoma overview Oral Oncol 45 301–308 https://doi.org/10.1016/j.oraloncology.2009.01.004 PMID: 19249237
6. Rogers SN, Brown JS, and Woolgar JA, et al (2009) Survival following primary surgery for oral cancer Oral Oncol 45 201–211 https://doi.org/10.1016/j.oraloncology.2008.05.008
7. Patil VM, Noronha V, and Joshi A, et al (2020) Chemoradiation in unresectable oral cavity cancer: a myth or reality! South Asian J Cancer 9(4) 195–198 https://doi.org/10.1055/s-0041-1728225
8. Kalavrezos N and Bhandari R (2010) Current trends and future perspectives in the surgical management of oral cancer Oral Oncol 46 429–432 https://doi.org/10.1016/j.oraloncology.2010.03.007 PMID: 20381408
9. Sieczka E, Datta R, and Singh A, et al (2001) Cancer of the buccal mucosa: are margins and T-stage accurate predictors of local control? Am J Otolaryngol 22 395–399 https://doi.org/10.1053/ajot.2001.28067 PMID: 11713724
10. Yousem DM, Gad K, and Tufano RP (2006) Resectability issues with head and neck cancer AJNR Am J Neuroradiol 27 2024–2036 PMID: 17110661 PMCID: 7977212
11. Posner MR, Hershock DM, and Blajman CR, et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer N Engl J Med 357 1705–1715 https://doi.org/10.1056/NEJMoa070956 PMID: 17960013
12. Vermorken JB, Remenar E, and Van Herpen C, et al (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer N Engl J Med 357 1695–1704 https://doi.org/10.1056/NEJMoa071028 PMID: 17960012
13. Liao C, Ng S, and Chang J, et al (2007) T4b oral cavity cancer below the mandibular notch is resectable with a favourable outcome Oral Oncol 43 570–579 https://doi.org/10.1016/j.oraloncology.2006.06.008
14. Liao C-T, Chang JT-C, and Wang H-M, et al (2006) Surgical outcome of T4a and resected T4b oral cavity cancer Cancer 107 337–344 https://doi.org/10.1002/cncr.21984 PMID: 16770782
15. Liao C-T, Huang S-F, and Chen I-H, et al (2008) When does skin excision allow the achievement of an adequate local control rate in patients with squamous cell carcinoma involving the buccal mucosa? Ann Surg Oncol 15 2187–2194 https://doi.org/10.1245/s10434-008-9980-4 PMID: 18506533
16. Patil VM, Noronha V, and Joshi A, et al (2013) Induction chemotherapy in technically unresectable locally advanced oral cavity cancers: does it make a difference? Indian J Cancer 50(1) 1–8 https://doi.org/10.4103/0019-509X.112263 PMID: 23713035
17. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136 E359–E386 https://doi.org/10.1002/ijc.29210
18. Liao C-T, Chang JT-C, and Wang H-M, et al (2006) Surgical outcome of T4a and resected T4b oral cavity cancer Cancer 107 337–344 https://doi.org/10.1002/cncr.21984 PMID: 16770782
19. Patil VM, Prabhash K, and Noronha V, et al (2014) Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers Oral Oncol 50(10) 1000–1004 https://doi.org/10.1016/j.oraloncology.2014.07.015 PMID: 25130412
20. Paccagnella A, Orlando A, and Marchiori C, et al (1994) Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo J Natl Cancer Inst 86(4) 265–272 https://doi.org/10.1093/jnci/86.4.265 PMID: 8158680
21. Haddad R, O’Neill A, and Rabinowits G, et al (2013) Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial Lancet Oncol 14(3) 257–264 https://doi.org/10.1016/S1470-2045(13)70011-1 PMID: 23414589
22. Joshi P, Patil V, and Joshi A, et al (2013) Neo-adjuvant chemotherapy in advanced hypopharyngeal carcinoma Indian J Cancer 50(1) 25–30 https://doi.org/10.4103/0019-509X.112286 PMID: 23713041
23. Salama JK, Stenson KM, and Kistner EO, et al (2008) Induction chemotherapy and concurrent chemoradiotherapy for locoregionally advanced head and neck cancer: a multi-institutional phase II trial investigating three radiotherapy dose levels Ann Oncol Off J Eur Soc Med Oncol ESMO 19(10) 1787–1794 https://doi.org/10.1093/annonc/mdn364
24. Zhong L, Zhang C, and Ren G, et al (2013) Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma J Clin Oncol Off J Am Soc Clin Oncol 31(6) 744–751 https://doi.org/10.1200/JCO.2012.43.8820
25. Licitra L, Grandi C, and Guzzo M, et al (2003) Primary chemotherapy in resectable oral cavity squamous cell cancer: a randomized controlled trial J Clin Oncol Off J Am Soc Clin Oncol 21(2) 327–333 https://doi.org/10.1200/JCO.2003.06.146
26. Bernier J, Cooper JS, and Pajak TF, et al (2005) Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck 27(10) 843–850 https://doi.org/10.1002/hed.20279 PMID: 16161069
27. Bernier J, Domenge C, and Ozsahin M, et al (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer N Engl J Med 350(19) 1945–1952 https://doi.org/10.1056/NEJMoa032641 PMID: 15128894






