Outcomes of clinico-radiologically advanced cancer (cT4b) of buccal mucosa: a retrospective analysis of 104 patients
Suvraraj Das1, Rajeev Sharan2, Anoop Attakkil2, Saugata Sen3, Kapila Manikantan2, Prateek V Jain2 and Pattatheyil Arun2
1Department of Surgical Oncology, Tata Medical Center, Kolkata 700160, India
2Department of Head and Neck Surgery, Tata Medical Center, Kolkata 700160, India
3Department of Radiology, Tata Medical Center, Kolkata 700160, India
Abstract
Although guidelines recommend non-surgical management for cT4b patients, recent studies have shown that upfront surgery in carefully selected patients can be performed with acceptable long-term survival benefit. In this study, we analysed the survival outcome of curative intent treatment on cT4b patients. Data from 104 patients who were staged cT4b and underwent upfront surgery for squamous cell carcinoma of buccal mucosa were retrospectively analysed. Outcome measures were locoregional recurrence-free survival (LRFS), disease-free survival (DFS) and overall survival (OS). The study cohort comprised 104 patients who had a median age of 52.5 years (range 27–81 years) and included 81 males (77.9%). Thirty-six patients had masticator space involvement on final histopathology, designating them as pT4b. Contrast enhanced computed tomography scan demonstrated 91.67% sensitivity in identifying masticator space involvement, albeit with a lower accuracy of 31.7%. Pathologically, clear margins were achieved in 79 (76%) patients. 62 (59.7%) and 20 (19.2%) patients received adjuvant radiotherapy (RT) and adjuvant chemoradiotherapy respectively. 2-year LRFS, DFS and OS were 61.8%, 60% and 68.1%, respectively. On multivariate analyses, involved margins (hazard ratio (HR) 28.766, p = 0.006), pN2b status (HR 4.68, p = 0.027) and perineural invasion (PNI) (HR 3.001, p = 0.027) showed statistically significant impact on LRFS, involved margins (HR 28.859, p = 0.008) and pN2b status (HR 4.018, p = 0.004) affected DFS. Involved margins (HR 14.139, p = 0.023) and pN2b status (HR 3.166, p = 0.025) showed statistically significant impact on OS. In conclusion, upfront surgery is a feasible option for patients with carcinoma of the buccal mucosa with the involvement of the masticator space. Survival outcomes are better in patients where resection is achieved with clear margins, and regional disease is limited to a single cervical lymph node.
Keywords: squamous cell carcinoma of head and neck, locoregional recurrence free survival, disease free survival, overall survival, CECT scan, cT4b lesions
Correspondence to: Prateek V Jain
Email: prateek.jain@tmckolkata.com and prateekjain70@gmail.com
Published: 26/05/2022
Received: 08/08/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Almost two-thirds of all cancers in the oral cavity are present in advanced stages in the Indian subcontinent [1]. Compared to western countries where the tongue is the commonest site or origin, the gingivo-buccal mucosa predominates as the most prevalent subsite in our patients where consumption of betel nut, tobacco and areca nut have been implicated as aetiological factors [2]. Moreover, the proximity of the buccal mucosa to the masticator space and pterygoid plates results in early invasion of these structures, escalating the stage to T4b [3].
Contrast enhanced computed tomographic (CECT) scan of the neck remains the standard pre-operative imaging modality for malignancy of the buccal mucosa. Although CECT scan with 1.25 mm sections has shown to be highly sensitive and specific (sensitivity 82.6%, specificity 86.9%) for identification of mandibular invasion, its accuracy in diagnosing masticator space invasion has not been established so far [4].
Current National Comprehensive Cancer Network guidelines recommend clinical trials or non-surgical management for T4b disease in view of very advanced stage of the disease, difficulty in achieving R0 resection and increased surgical morbidity [5]. However, after the results published by Liao et al [6] showing favourable outcomes in infra-notch cT4b patients, surgical resection for selected patients has been the norm. Trivedi [7] have also proposed radical compartmental en-bloc resection in T4b disease to attain clear margins. However, extension to the infratemporal fossa defies attempts to delineate margins with accuracy as the area is bounded by bone on three sides and does not yield itself to clinical examination on the table and the surgeon is obligated to depend entirely on the accuracy of imaging modalities to guide his surgery on.
In this study, we analysed the outcomes of curative intent treatment for cT4b patients in terms of locoregional recurrence-free survival (LRFS), disease-free survival (DFS) and overall survival (OS).
Material and methods
This retrospective cohort study comprised patients who underwent surgery for oral squamous cell carcinoma (OSCC) from August 2011 to June 2017. Patients who underwent tumour resection as the primary method of treatment and had primary tumour staged T4b clinico-radiologically or on definitive histopathology were considered eligible for inclusion. Patients who had primary lesions staged other than T4b had history of receiving prior treatment or if their preoperative CECT scan was unavailable were excluded from the study. A total number of 725 patients of OSCC were operated on in this period, of which 104 eligible patients formed the cohort for this study. Approval from the Institutional Ethical and Review committee was obtained prior to commencing the study vide Ref No EC/WV/TMC/19/21.
Demographic and tumour characteristics, adjuvant treatment, and clinical outcomes were taken directly from patient charts. In all cases, preoperative CECT scan of the neck was evaluated by a senior radiologist and was divided into two groups, viz., with or without involvement of masticator space (involvement of at-least one of masseter, medial pterygoid, lateral pterygoid or temporalis muscle). The radiologist was blinded to the final histopathology report.
Other study variables, namely, age, gender, tumour grade (well differentiated, moderately differentiated and poorly differentiated) pathological T classification and N classification, extra nodal extension (ENE), lympho-vascular invasion (LVI) and perineural invasion (PNI), were derived from definitive pathology reports, Information on adjuvant treatment with radiation or chemotherapy (any dosage or type of radiotherapy (RT) or chemo-radiation (CTRT) was recorded as ‘yes’ or ‘no’). Staging was based on the the American Joint Committee on Cancer (edition 7) [8] histopathologic risk factors, namely, pathological T and N classification, tumour site, tumour grade, ENE, LVI, PNI and surgical margin were considered for administration of adjuvant treatment by a multidisciplinary team.
Clinical outcomes were retrieved from the patient charts. LRFS was defined as the time between the day of primary surgery for OSCC and the date of pathological confirmation of locoregional disease recurrence. LRFS for patients without documentation of locoregional recurrence was censored on the last day of their follow up. DFS was defined as the interval between day of primary surgery for OSCC and the day of clinico-radiological confirmation of disease recurrence (locoregional or distant metastasis). DFS for patients without documentation of disease recurrence was censored at the last day of follow up. Death was also considered as an event for calculation of DFS and LRFS. The time for OS was determined between the day of primary surgery for OSCC and the date of death. OS for patients without documentation of death was censored at the last date that the patient had been known to have been alive.
Data analysis was done using Stata version 14.2 (Stata Corp). Kaplan–Meier survival curves were used to calculate LRFS, DFS and OS. Comparisons survival between groups were performed using Log-rank Tests. Multivariate Cox proportional hazards models were used to assess the prognostic effects of demographic and tumour characteristics and adjuvant treatment on survival outcomes. Variables with univariate p-values < 0.05 were included in multivariate models. Variable selection using both forward selection (p ≤ 0.05 to enter) and backward elimination (p > 0.05 to remove) was performed to obtain final models. The level of statistical significance for all tests was < 0.05.
Results
The study cohort comprised 104 patients of which 81 patients (77.9%) were male and 23 were female (22.1%). The age of the patients ranged between 27 and 81 years, with a median age of 52.5 years. Median follow up of all the patients who were alive at the time of analysis was 28 months (range 0.5–76.5 months)
All patients underwent a CECT scan, which revealed 101 (97.1%) patients to have masticator space involvement. All patients had infra-mandibular notch disease on pre-operative CECT scan. 33 patients had masticator space involvement on which was corroborated with HPR. Likewise, there were three cases where masticator space was free on CECT scan, but final histopathology reported involvement of the masticator space. CECT scan identified the masticator pace involvement with 91.67% sensitivity with a negative predictive value of 91.67% (Table 1).
Histopathological examination revealed majority of the patients to have moderately differentiated tumours (74.6%), followed by poorly differentiated (19.6%) and well differentiated ones (7.8%). 2 patients (1.9%) had involved margins, whereas 23 patients (22.1%) had close margins. 56 patients had bone involvement out of which 9 had superficial erosion and 47 patients (45.2%) had bone destruction. 36 patients (34.6%) had masticator space involvement (involvement of at least one of masseter, medial pterygoid, lateral pterygoid or temporalis muscle). Table 2 depicts all clinicopathological prognostic factors and unadjusted ‘p’ values on log rank test showing their impact of LRFS, DFS and OS.
After the multidisciplinary team discussion, 22 patients (21.1%) were kept on follow up, 62 patients (59.7%) received adjuvant RT whereas 20 patients (19.2%) received adjuvant CTRT.
2-year LRFS was 61.8% (95% confidence interval (CI) 51.0%–70.9%) and 5-year LRFS was 52.5% (95% CI = 40.5%–63.1%) in this study. On adjusting for other prognostic indicators, involved margins after resection (hazard ratio (HR) 28.766, p 0.006), metastasis to more than one lymph node (HR = 4.68, p = 0.027) and PNI (HR = 3.001, p = 0.027) showed statistically significant impact on LRFS (Table 3).
We observed a 2-year DFS of 60% (95% CI = 49.3%–69.2%) and a 5-year DFS of 49.6% (95% CI = 37.9%–60.2%). On adjusting for other prognostic indicators, involved margins after resection (HR = 28.859, p = 0.008) and metastasis to more than one lymph node (HR = 4.018, p = 0.004) showed statistically significant impact on DFS (Table 3).
Table 1. Efficacy of CECT scan in identifying masticator space involvement.
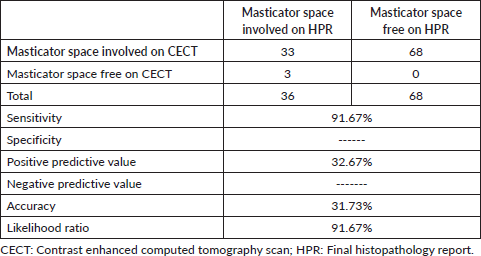
Table 2. Effect of demographic and histopathological parameters on LRFS, DFS and OS. (N = 104).
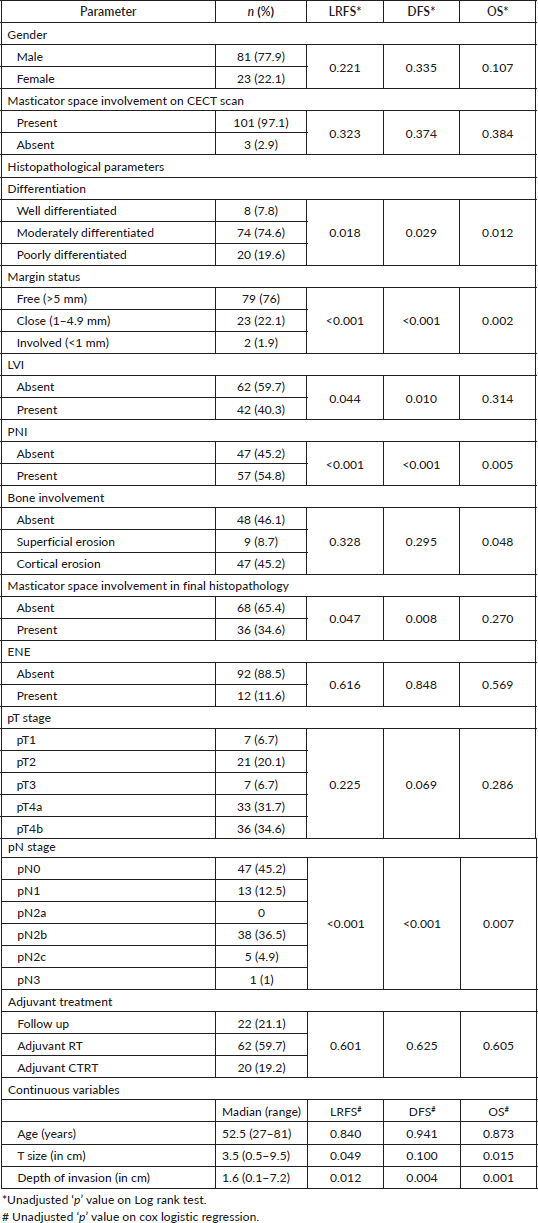
Table 3. Adjusted HR and their significance.
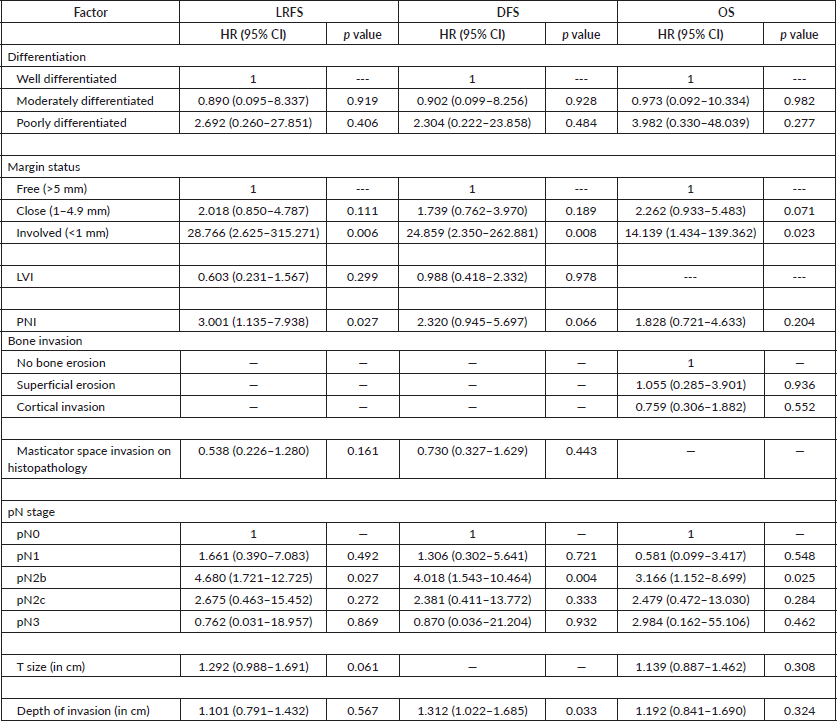
OS in this cohort at 2 and 5 years was 68.1% (95% CI = 57.0%–77.0%) and 58.6% (95% CI = 46%–69.2%), respectively. Involved margins after resection (HR = 14.139, p = 0.023) and metastasis to more than one lymph node (HR = 3.166, p = 0.025) showed statistically significant impact on OS (Table 3).
Proportion of close/ involved margins was higher in patients who had pathological involvement of masticator space (13.2% versus 44.4%, p = 0.002) (Table 4).
Patients diagnosed pT4b had a 2-year LRFS, DFS and OS (95% CI) of 49.3% (31.4%–64.8%), 44.5% (27.5%–60.2%) and 55.2% (35%–71.5%), respectively. 5-year survival could not be calculated.
Discussion
Squamous carcinomas of the oral cavity are staged as T4b when the disease invades the masticator space, pterygoid plates, or skull base and/or encases the internal carotid artery and is referred to as very advanced locoregional disease [8]. Although these tumours are generally considered unresectable, the surgeon can achieve clear margins with his surgery in a subset of T4b tumours depending on the location and surgical technique adopted. While disease encasing the internal carotid artery or invading the skull base are generally considered unresectable, disease confined to the masticator space below the mandibular notch are often considered resectable [6].
Table 4. Comparison of margin status in patients with masticator space involvement.
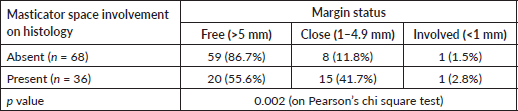
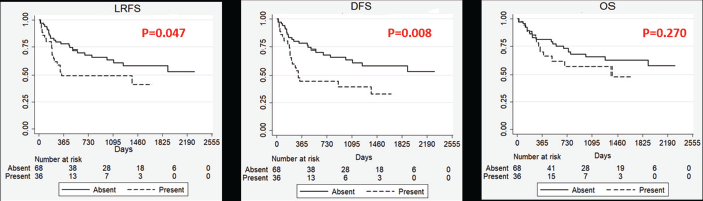
Figure 1. Kaplan–Meier Curves showing impact of masticator space involvement on survival outcomes. LRFS: Locoregional recurrence free survival, DFS: Disease free survival, OS: Overall survival, P: p value on log rank test. Absent: Masticator space not involved of histopathology, Present: Masticator space involved on histopathology (pT4b).
Anatomically, the masticator space is located behind the retromolar trigone. It is a pyramidal-shaped space with its base toward the skull base and apex at the lower border of the mandible [10]. There are two described ways tumour spread could occur in the masticator space – one by an upward spread along the neurovascular bundle and the other – through the medial side of the masticator space through the loose areolar tissue, buccal space, then to the lateral wall of the maxilla [11–13].
The decision to proceed with surgery in buccal mucosa malignancy is based on preoperative imaging. CECT scan is the preferred modality for buccal mucosa lesions as CECT is better than magnetic resonance imaging (MRI) in detecting subtle erosions of the cortex of the mandible and provides excellent detection and characterisation of the tumour matrix mineralisation [14, 15]. In our study, CECT scan was performed for all patients, and had a sensitivity of 91.67%, with a lower accuracy (31.73%) in identifying masticator space involvement. These findings contrasted with Huang et al [16] who demonstrated CECT to be 25% sensitive and 83.3% specific in identifying pterygoid muscle involvement. MRI had a superior sensitivity (50%) but a lower specificity (75%) when compared to the CECT scan in identifying pterygoid muscle involvement. However, the number of patients in the study was small. The addition of MRI to CECT scan may improve the identification of masticator space involvement. However, further studies are required to draw a meaningful conclusion. Huang et al [16] recommended fused fluorodeoxyglucose positron emission tomography (PET) and MRI as a more reliable imaging modality for focal invasion assessment and tumour size delineation in advanced OSCC compared to PET/CT, MRI and CECT.
Although protocols involving induction chemotherapy followed by surgery and/or CTRT have been recommended in a few studies, due to the lack of survival benefit and the extent of surgery after tumour reduction following chemotherapy, surgery followed by adjuvant radiation or CTRT remains the standard of care as curative-intent treatment. For T4b tumours surgery is technically challenging, leading to triaging many of these patients with non-surgical modalities [5, 9, 17–19]. However, recent publications have advocated initial surgery followed by adjuvant treatment for resectable T4b lesions. This is associated with a better 5-year DFS and OS [3]. Our study affirms this with a 5-year LRFS and DFS of 52.5% and 49.6%, respectively.
Mair et al [20] reported favourable disease outcomes in T4b buccal mucosa cancer patients treated by multimodality treatment of primary surgery and adjuvant RT or concurrent CTRT. The loco-regional control, DFS and OS were 68.2%, 54.7% and 48.7% respectively, which are comparable favourably with this study. Their cohort excluded patients with supra-notch disease. The study observed significant difference in oncologic outcome with respect to adequacy of surgical margin related to local control (49.6% versus 41.1%, p = 0.025) and disease-free survival (65.3% versus 42%, p = 0.035); confirming the importance of achieving uninvolved surgical margins when undertaking primary surgery.
Our study, in agreement with Mair et al [20] found out that the initial clear surgical margin of resection was favourably affecting LRFS, DFS and OS. In addition, metastasis to two or more cervical lymph nodes was associated with poorer survival outcomes.
Our study showed a poorer LRFS and DFS in patients with pathological involvement of masticator space (Figure 1). Also, patients with pathological involvement of masticator space showed higher proportion of patients with a close or involved margin in the postoperative specimen. This could be due to the inherent aggressive nature of these tumours or the unfavourable anatomic location deters the surgeon from reaching around the lesion to achieve clear margin in the masticator space.
Retrospective design and the small number of patients are the limitations of this study. The survival outcomes of pT4b patients would have yielded insightful information but were not analysed due to very small number of patients with true masticator space involvement. This highlights the need for a multicentre study to provide further evidence on treatment of patients with masticator space involvement.
Conclusion
Upfront surgery is a feasible option for patients with buccal mucosa malignancy with masticator space involvement. Survival outcomes are favourable in patients where resection with clear margins is achieved, and the regional disease limited to a single cervical lymph node.
List of Abbreviations
DFS Disease free survival
CECT scan Computed tomographic scan
CI 95% Confidence interval
CTRT Chemo-radiation
ENE Extra nodal extension
HR Hazard ratio
LRFS Locoregional recurrence free survival
LVI Lympho vascular invasion
MRI Magnetic resonance imaging
OS Overall survival
OSCC Oral squamous cell carcinoma
PNI Perineural Invasion
RT Radiotherapy
Conflicts of interest
None.
Funding
This study did not receive any external funding.
References
1. Mathur P, Sathishkumar K, and Chaturvedi M, et al (2020) Cancer Statistics, 2020: Report From National Cancer Registry Programme, India JCO Glob Oncol 6 1063–1075 https://doi.org/10.1200/GO.20.00122 PMID: 32673076 PMCID: 7392737
2. Pillai V, Yadav V, and Kekatpure V, et al (2019) Prognostic determinants of locally advanced buccal mucosa cancer: do we need to relook the current staging criteria? Oral Oncol 95 43–51 https://doi.org/10.1016/j.oraloncology.2019.05.021 PMID: 31345393
3. Liao CT, Wen YW, and Lee SR, et al (2017) Clinical outcomes of Taiwanese patients with cT4 oral cavity squamous cell carcinoma: toward the identification of the optimal initial treatment approach for cT4b patients Ann Surg Oncol 24 785–793 https://doi.org/10.1245/s10434-016-5629-x
4. Handschel J, Naujoks C, and Depprich RA, et al (2012) CT-scan is a valuable tool to detect mandibular involvement in oral cancer patients Oral Oncol 48 361–366 https://doi.org/10.1016/j.oraloncology.2011.11.009
5. Pfister DG, Spencer S, and Adelstein D, et al (2020) Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology J Natl Compr Canc Netw 18 873–898 https://doi.org/10.6004/jnccn.2020.0031 PMID: 32634781
6. Liao CT, Ng SH, and Chang JT, et al (2007) T4b oral cavity cancer below the mandibular notch is resectable with a favorable outcome Oral Oncol 43 570–579 https://doi.org/10.1016/j.oraloncology.2006.06.008
7. Trivedi NP (2018) Oral cancer involving masticator space (T4b): review of literature and future directions Head Neck 40 2288–2294 https://doi.org/10.1002/hed.25211 PMID: 29756367
8. Edge SB and Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM Ann Surg Oncol 17 1471–1474 https://doi.org/10.1245/s10434-010-0985-4 PMID: 20180029
9. Bossi P, Lo Vullo S, and Guzzo M, et al (2014) Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial Ann Oncol 25 462–466 https://doi.org/10.1093/annonc/mdt555 PMID: 24401930
10. Fernandes T, Lobo JC, and Castro R, et al (2013) Anatomy and pathology of the masticator space Insights Imaging 4 605–616 https://doi.org/10.1007/s13244-013-0266-4 PMID: 23888350 PMCID: 3781239
11. Kowalski LP, Hashimoto I, and Magrin J (1993) End results of 114 extended “commando” operations for retromolar trigone carcinoma Am J Surg 166 374–379 https://doi.org/10.1016/S0002-9610(05)80336-8 PMID: 8214296
12. Antoniades K, Lazaridis N, and Vahtsevanos K, et al (2003) Treatment of squamous cell carcinoma of the anterior faucial pillar-retromolar trigone Oral Oncol 39 680–686 https://doi.org/10.1016/S1368-8375(03)00077-0 PMID: 12907207
13. Pinsolle J, Demeaux H, and Coustal B, et al (1992) Results of surgical treatment of T3 and T4 tumors of the oral cavity and oropharynx Am J Surg 164 587–591 https://doi.org/10.1016/S0002-9610(05)80712-3 PMID: 1463105
14. Galli F, Flor N, and Villa C, et al (2010) The masticator space. Value of computed tomography and magnetic resonance imaging in localisation and characterisation of lesions Acta Otorhinolaryngol Ital 30 94–99 PMID: 20559479 PMCID: 2882142
15. Som PM and Curtin HD (2003) Parapharyngeal and masticator space lesions Head and neck imaging 4th edn, vol 2, eds PM Som, and HD Curtin (Mosby, St Louis) pp 1954–2003
16. Huang SH, Chien CY, and Lin WC, et al (2011) A comparative study of fused FDG PET/MRI, PET/CT, MRI, and CT imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma Clin Nucl Med 36 518–525 https://doi.org/10.1097/RLU.0b013e318217566f PMID: 21637051
17. Liao CT, Wang HM, and Ng SH, et al (2006) Good tumor control and survivals of squamous cell carcinoma of buccal mucosa treated with radical surgery with or without neck dissection in Taiwan Oral Oncol 42 800–809 https://doi.org/10.1016/j.oraloncology.2005.11.020 PMID: 16458038
18. Zhong LP, Zhang CP, and Ren GX, et al (2013) Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma J Clin Oncol 31 744–751 https://doi.org/10.1200/JCO.2012.43.8820
19. Joshi A, Patil VM, and Noronha V, et al (2013) Is there a role of induction chemotherapy followed by resection in T4b oral cavity cancers? Indian J Cancer 50 349–355 https://doi.org/10.4103/0019-509X.123627 PMID: 24369216
20. Mair MD, Sawarkar N, and Nikam S, et al (2018) Impact of radical treatments on survival in locally advanced T4a and T4b buccal mucosa cancers: Selected surgically treated T4b cancers have similar control rates as T4a Oral Oncol 82 17–22 https://doi.org/10.1016/j.oraloncology.2018.04.019 PMID: 29909893






