Measuring patient-reported outcomes in advanced gastric cancer
Jianming Xu1, TR Jeffry Evans2, Cheryl Coon3, 4, Kati Copley-Merriman5 and Yun Su6
1 Beijing 307 Hospital Cancer Center, Beijing 100071, China
2 University of Glasgow, Beatson Institute for Cancer Research, Garscube Estate, Switchback Road, Bearsden, Glasgow G61 1BD, UK
3 RTI Health Solutions, 200 Park Offices Drive, Research Triangle Park, NC 27709, USA
4 Current address: Adelphi Values, 290 Congress Street, Boston, MA 02210, USA
5 RTI Health Solutions, 3005 Boardwalk Street, Suite 105, Ann Arbor, MI 48108, USA
6 Bristol-Myers Squibb, 5 Research Parkway, Wallingford, CT 06492, USA
Correspondence to: Cheryl D. Coon. Email: cheryl.coon@adelphivalues.com
Abstract
Background: Gastric cancer (GC), one of the most common cancers in the world, is often diagnosed at an advanced stage and associated with a poor prognosis. Quality of life and patient-reported outcomes (PROs) are important considerations when treating GC patients. The aim of this study was to identify existing PRO instruments that would be appropriate for use in GC trials.
Methods: Data were obtained from a systematic literature review and interviews with clinical experts. A literature search was conducted using OVID (EMBASE and MEDLINE) and yielded 1,008 abstracts; 92 assessed PROs in an advanced GC.
Results: Key symptoms and functional impacts identified through the literature and expert input included abdominal pain or pain at the site of distant metastases, dysphagia and other symptoms related to eating, and digestive symptoms. The liver and lungs were the most frequent locations of metastases, leading to dyspnea, abdominal fullness, and jaundice. Symptoms related to changes in bowel habits appeared to be more frequent and pronounced in Asian patients, possibly due to the higher prevalence of GC in the body of the stomach in this population. The five most commonly used PRO instruments were identified, but their validity in advanced-stage GC patients remains unclear.
Conclusions: The symptoms and functional impacts identified here should be confirmed with robust input from advanced-stage GC patients. Optimal measurement of PROs in GC should account for patient burden and possible differences between Asian and non-Asian patients.
Keywords: gastric cancer, patient outcome assessment, outcome measures, digestive signs, symptoms.
Introduction
Gastric cancer (GC) and oesophagogastric junction (OGJ) adenocarcinoma are among the leading causes of cancer mortality in the world (736,000 deaths in 2008), accounting for approximately 24,000 and 10,400 newly diagnosed cancers each year in the United States and the United Kingdom, respectively [1]. Although there has been a decline in the incidence of GC in Western countries over the past few decades, this decline has been accompanied by an increase in the incidence of tumours of the OGJ and a shift toward poorly differentiated adenocarcinomas [2].
There is a significant geographical variation in the incidence of GC, with China having the highest incidence rate worldwide [3], resulting in 352,000 deaths in 2008 [4]. More than half of all the cases in the world occur in Western Pacific countries, including Australia, China, Japan, Korea, and Vietnam. The early stages of GC are often asymptomatic. The most common symptoms at diagnosis include abdominal pain, dysphagia, weight loss, nausea, and vomiting [5, 6], and the symptoms of advanced GC are often indistinguishable from benign disorders. Thus, the diagnosis is frequently made when the cancer is at an advanced stage, which is associated with a poor prognosis [7]. The estimated five-year survival rate is just 7% in patients with stage-IV disease [8].
The vast majority (80–90%) of patients present with locally advanced or metastatic disease that is unsuitable for curative resection. Combination chemotherapy results in a significant survival advantage in patients with advanced GC when compared with the best supportive care in randomised clinical trials [9–11]. There is currently no chemotherapy regimen that is universally accepted as a standard of care worldwide. The most frequently used regimens are either doublets of platinum with a fluoropyrimidine or a triplet drug regimen combining either epirubicin or docetaxel with a platinum and a fluoropyrimidine [12–14]. Nevertheless, the overall survival (OS) remains disappointing in these patients, with a median of 10–11 months [14], although the addition of trastuzumab to a chemotherapy backbone has further improved the OS to beyond one year in the subset of patients with HER2-positive tumours [15]. Consequently, quality of life (QOL) remains an important consideration when planning treatment in patients with an advanced disease.
Assessment of QOL, symptoms, and other related patient-reported outcomes (PROs) is increasingly performed in oncology clinical trials. In patients with advanced disease, the goals of the treatment are to achieve palliative benefits and improvement in survival. Therefore, it is crucial to understand the impact of treatment on symptoms, functioning, and QOL. Understanding the benefit–risk profile associated with oncology treatments is also of increasing importance to regulatory agencies, and PRO assessment is an important source of information regarding the positive and negative impacts of treatment from the patient’s perspective. Within a clinical trial setting, data on symptoms and symptom burden through PRO instruments can supplement the understanding of treatment benefits provided by the efficacy outcomes such as OS, progression-free survival (PFS), and response rate. PRO data may provide evidence of improvement in QOL or functional status related to reduced symptoms, even in the absence of improvement in the efficacy outcomes.
A survey of oncologists found that 51% of the respondents reported that PRO study findings had influenced their recommendations for treatment [16]. From the patient’s perspective, they are becoming more involved in decision making for their own treatment and subsequent treatment compliance. This trend is particularly relevant as an increasing number of oncology therapies are administered orally at home, and noncompliance with treatment may be a significant cause of treatment failure [17]. Finally, baseline PRO instruments have been shown to predict survival in many cancer types, including advanced GC [18]. Consequently, the aim of this study was to identify the existing PRO instruments that would be appropriate to apply in clinical trials in patients with advanced or metastatic GC.
Methods
A structured literature search with predefined inclusion criteria was conducted using OVID, which accesses EMBASE, OVID MEDLINE, and EconLit. The search strategy combined disease terms for advanced metastatic stage-III or -IV GC or stomach cancer, with terms describing the outcomes of interest, including QOL, patient perspective, preference, patient satisfaction, activities of daily living, burden of illness, symptom, PROs, well-being, pain, functional status, and health status. The search was conducted in 2011 with no limits on publication date, study type, or language; 1,008 articles were identified. Abstracts of all 1,008 articles were reviewed, and those articles deemed relevant were retrieved for full text review. From the 1,008 abstracts reviewed, 92 relevant studies met the inclusion criteria and were included in this review. Studies were considered relevant to this review if they described the development, validation, or use of a PRO instrument in a chemotherapy clinical study or an observational study in advanced GC. PRO instruments described in these studies included assessments of QOL, functional status, health status, treatment satisfaction, or GC symptoms.
Additional potentially relevant studies were identified from the reference lists of the retrieved articles. Information on the association of GC symptoms and tumour location was informed by the clinical experience of the authors. Further information about PRO instruments used to assess outcomes in GC was obtained from the summaries of GC treatments as described in the Physician’s Desk Reference, the European Medicines Agency’s summary of product characteristics, and clinicaltrials.gov.
Results
Key symptoms and functional impacts
Key symptoms and functional impacts identified through the literature and expert input included abdominal pain or pain from metastatic disease sites, dysphagia and other eating-related symptoms, and digestive symptoms. The metastases in different sites and extents usually lead to different symptoms, such as dyspnea, abdominal fullness, and occasionally, jaundice, etc. Symptoms related to the change in bowel habit appeared to be more frequent and pronounced in Asian patients, possibly due to a higher prevalence of GC in the body of the stomach in this population.
Association of patient-reported symptoms, location of GC tumour, and geographic location
Regional differences in the location of GC tumours have been noted in many studies and in clinical practice. Proximal GC is more common in Western countries and is associated with poorer outcomes [19]. While the incidence of GC in general is declining around the world, this is generally limited to cancers of the cardia [20]. In contrast, distal tumours are more common in Eastern countries, and it has been speculated that this incidence rate is associated with food additives consumed more frequently in Asian cultures [20, 21]. These patterns also hold for ethnic groups within the United States, with cancers of the cardia being more common in whites and cancers of the antrum and pylorus being more common in African Americans and Asian and Pacific Islanders [22]. Geographic regions can be considered predictors of safety outcomes, such as neutropaenia and diarrhoea, which have been shown to have lower incidence rates in Asian trials versus non-Asian trials [23].
PRO instruments available for use in advanced GC
Despite the use of the PRO instruments in advanced-staged GC clinical trials in the published literature, no product label in the Physician’s Desk Reference for a GC treatment referenced a PRO instrument at the time this review was conducted. A search of the European Medicines Agency’s summary of product characteristics identified four products, all docetaxel based, that are indicated for advanced-stage GC and that referenced a PRO instrument [specifically, the European Organisation for Research and Treatment QOL Questionnaire-Core 30 (EORTC QLQ-C30)]. Although these four products are indicated for advanced-stage GC, the EORTC QLQ-C30 was used only in docetaxel-based studies in metastatic breast cancer. Thus, the sensitivity of the instrument in measuring treatment differences for GC cannot be determined from these products’ labels.
A search of clinicaltrials.gov identified 97 studies in advanced-stage GC that used a PRO instrument, and 17 PRO instruments specific to GC were identified. Combined with the results of previously published literature, the PRO instruments reported to be used in a GC population were as follows:
(a) Health-related QOL: SF-36 Health Survey and the McGill QOL.
(b) General cancer: EORTC QLQ-C30.
(c) GC specific: European Organisation for Research and Treatment QOL Questionnaire-GC Module (EORTC QLQ-STO22).
(d) Other cancer specific: European Organisation for Research and Treatment QOL Questionnaire-Oesophageal, European Study for Pancreatic Cancer-QOL Questionnaire, the MD Anderson Symptom Inventory-Gastrointestinal (MDASI-GI), and Functional Assessment of Cancer Therapy (FACT)-Oesophageal.
(e) Cancer treatment: QOL-Anticancer Drugs.
(f) Symptoms: FACT-Fatigue, FACT-Anaemia, Brief Pain Inventory, and Edmonton Symptom Assessment Scale Score.
(g) Emotional functioning: Hospital Anxiety and Depression Scale, State–Trait Anxiety Inventory, Depression Status Inventory, Positive and Negative Affect Schedules, and Profile of Mood States.
(h) Other: Medical Coping Modes Questionnaire, Linear Analogue Self-Assessment, and Time Without Symptom and Toxicity.
(i) Utility: EuroQol-5 Dimension (EQ-5D) and Health Utilities Index 3.
Sensitivity of PRO instruments identified in the literature
The EORTC QLQ-C30 has been used frequently to assess outcomes in a GC population. This instrument has been able to demonstrate treatment differences or improvements on physical, role, cognitive, emotional, and social functioning, as well as fatigue, nausea and vomiting, pain, dyspnea, diarrhoea, constipation, insomnia, appetite loss, numbness or paraesthesia, and global health status. Treatment differences or improvements have also been shown in QOL on the EQ-5D visual analogue scale (VAS) and in anxiety and depression as reported on the Hospital Anxiety and Depression Scale. In addition to their use in clinical trials, many of the PRO instruments listed here were included in the observational or cross-sectional studies to predict outcomes, rather than to distinguish between treatments or assess efficacy within the treatment.
In the published psychometric evaluations, the instruments registered differences between groups that were known to differ on clinical outcomes. The EORTC QLQ-STO22 was able to distinguish between clinically distinct groups (curative versus palliative) on dysphagia, eating restrictions, taste problems, and body image [24]. The FACT-Gastric Cancer (FACT-Ga) showed significant differences between groups identifying themselves as having improved, worsened, or remained the same over a three-month study [25]. The MADSI-GI has been shown to be sensitive enough to distinguish the functional status in GI cancer patients and to differentiate among GC groups defined by disease level [26]. Finally, in one study, all the scales and items from the EORTC QLQ-C30 were able to discriminate among GC survivors, GC patients undergoing curative treatment and those undergoing palliative treatment, and GC patients categorised by the performance status [27].
Breadth of concepts measured
During the course of the review, five cancer-specific PRO instruments were identified as being potentially useful in assessing the treatment benefit in clinical trials of advanced GC: the EORTC QLQ-C30, the EORTC QLQ-STO22, the FACT-General (FACT-G), the FACT-Ga, and the MDASI-GI. These instruments were selected because of their demonstrated psychometric properties and evidence of appropriate patient involvement in the generation or confirmation of the questionnaire items—except the FACT-G, which was selected because of its intended use in combination with the FACT-Ga. Patient involvement in the development process supports an instrument’s face and content validity, in line with the recommendations of the US Food and Drug Administration’s guidance for PROs [28].
Three instruments were GC or GI cancer specific: the EORTC QLQ-STO22 [29], the FACT-Ga [30], and the MDASI-GI [26]. Two instruments were developed for cancer in general, to be used in conjunction with cancer-type-specific instruments: the EORTC QLQ-C30 [31] and the FACT-G [32].
The EORTC QLQ-STO22 and the FACT-Ga were specifically developed for patients with GC. The EORTC QLQ-STO22 was generated from a comprehensive literature search and physician interviews into a set of candidate concepts. Interviews conducted by the instrument developers with 24 health professionals and 58 GC patients (from a range of European countries, disease stages, and treatments) provided feedback on the scope of concepts represented, and cognitive debriefing with 115 additional GC patients tested the wording and appropriateness of the items. The FACT-Ga was developed from patient inputs (along with physician input) solicited for item generation, scale construction, content validation, and scale refinement [30]. While the patient characteristics for the initial patient input work of the FACT-Ga were not reported, later cognitive interviews in Canada and Japan included patients with GC and cancer of the GC cardia.
The EORTC QLQ-C30 and FACT-G, on the other hand, were developed for use in a broad range of cancer patients. The authors of the EORTC QLQ-C30, Blazeby et al [24], received positive feedback during their cognitive interviews with GC patients for the EORTC QLQSTO22. There is no evidence of content validity for the FACT-G in GC. Because the MDASI-GI is intended for GI cancer in general, it does not limit patient involvement to those with GC. However, the authors used a large group of patients with GI cancer to develop the instrument (item generation, item reduction, and cognitive debriefing), including 23 patients specifically with GC.
The two generic cancer instruments, EORTC QLQ-C30 and FACT-G, assess PROs related to well-being and physical, emotional, and social functioning. The GC-specific instruments, EORTC QLQ-STO22 and FACT-Ga, assess GC symptoms of pain, dysphagia, problems with eating, and reflux or upper GI symptoms. The MDASI-GI assesses GI symptoms, general cancer symptoms, and interference.
In addition to measuring aspects of health-related QOL, the five PRO instruments measure a number of GC symptoms. Table 1 provides a list of symptoms addressed in these instruments, listed as they would be used in practice (core plus tumour-specific module, where appropriate). None of the instruments captures the full range of GC symptoms, but the inclusion of the GC-specific modules (EORTC QLQSTO22 and FACT-Ga) and the more general GI-specific instrument (MDASI-GI) allow for a wide portion of symptoms to be covered. GC symptoms not present in any of the identified PRO instruments included haematemesis or melaena, changes in bowel habits, cough or haemoptysis, fever or sweats, pruritus, jaundice, need to eat frequent small meals, and bone pain.
Table 1. Overview of evaluated PRO instruments for advanced-stage GC: symptoms.
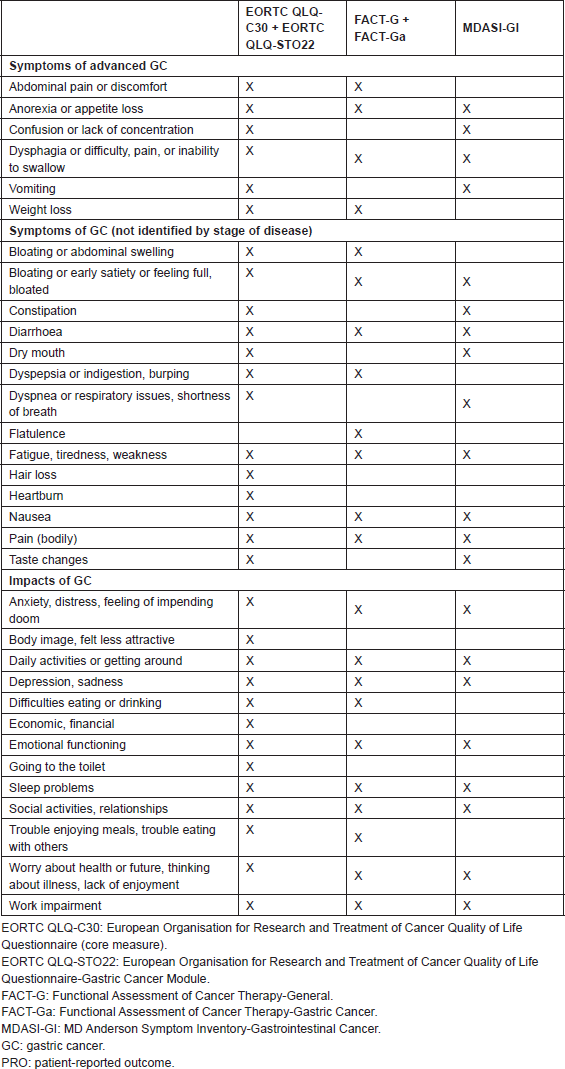
None of the identified PRO instruments comprehensively cover all of the QOL and symptom concepts that could be relevant to patients with advanced GC. The most comprehensive coverage of GC symptoms and their potential impact is provided by the EORTC QLQ-C30 supplemented with the EORTC QLQ-STO22; however, these two instruments combined have 52 items, which is likely to be burdensome for patients with advanced or metastatic GC. Perhaps because of the number of items in the pair of instruments, the randomised clinical trials in advanced GC that we identified used only the EORTC QLQ-C30 for assessing all the cancer types. This approach suggested that these trials did not assess concepts that may be important to patients with GC. Given these limitations and the content of the MDASI-GI, the 24-item MDASI-GI is a reasonable option that covers general cancer symptoms, relevant GI symptoms, and interference; also, the MDASI-GI can be completed by most of the patients in 2–3 min.
In terms of using these instruments in patients with advanced-stage GC, those instruments developed for use in GC studied patients with a range of disease stages, give breadth of coverage of the concepts. Thus, while the items may not have been targeted to advanced-stage GC patients specifically, the instruments should be appropriate for measuring a wide variety of disease states, which is appropriate for assessing change over time in this population.
Psychometric measurement properties
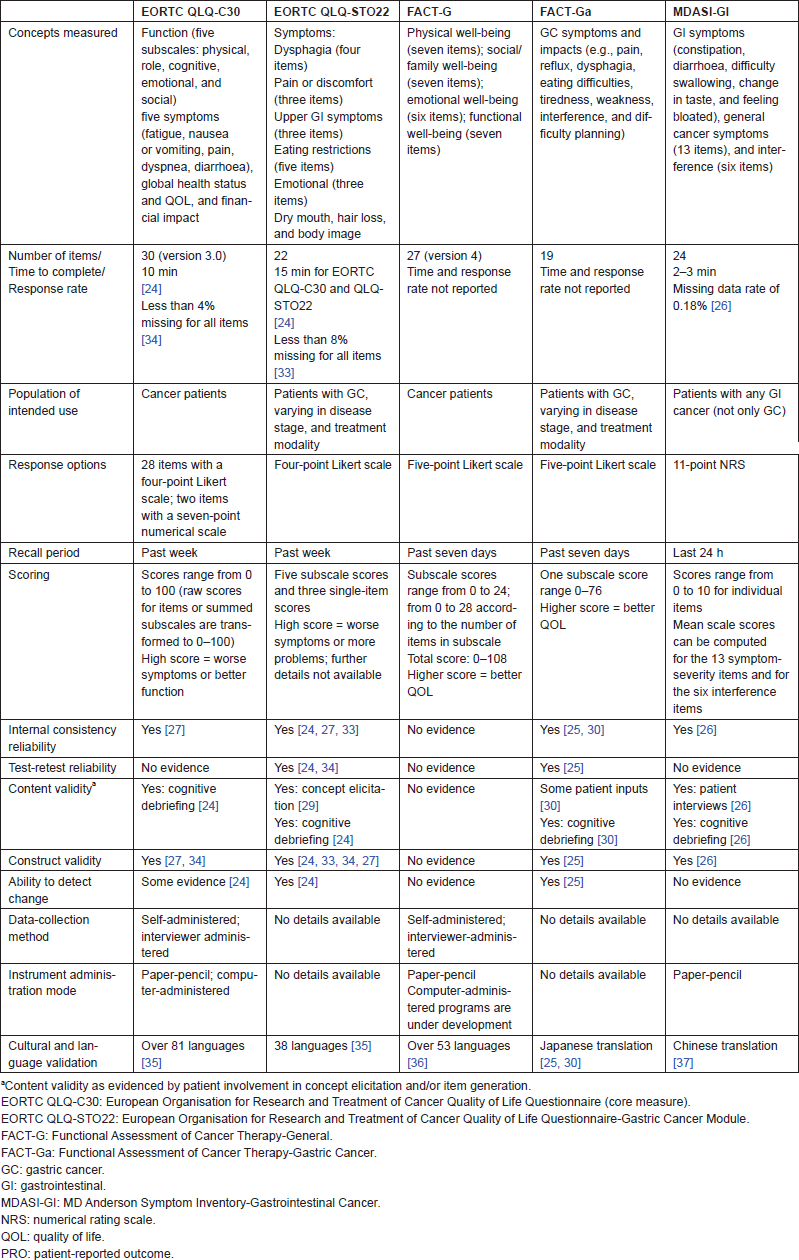
Table 2 provides an overview of the five instruments selected for the possible use in GC studies, on the basis of each instrument’s characteristics and the psychometric evidence it identifies. In some of the instruments, it should be noted that the psychometric properties were evaluated in patients with GC but the samples were not limited to only those with advanced-stage GC.
Table 2 shows that four of the five PRO instruments for use in GC demonstrated some levels of psychometric validity in a GC population, while the psychometric properties of the FACT-G have yet to be assessed (the instrument was included to complement the GC module, the FACT-Ga). Both internal consistency reliability and test–retest reliability have been demonstrated by the EORTC QLQ-STO22 and FACT-Ga in GC, while only internal consistency reliability has been demonstrated by the MDASI-GI and EORTC QLQ-C30 in GC. Each of the four instruments has documented validity (construct, discriminant, concurrent, or criterion), and the EORTC QLQ-STO22, the EORTC QLQ-C30, and the FACT-Ga have assessed ability to detect change in a GC population. In the studies that reported response rates, missing data did not appear to be a problem for the EORTC QLQ-C30, the EORTC QLQ-STO22, or the MDASI-GI.
Table 2. Overview of evaluated PRO instruments in advanced-stage GC: characteristics and psychometric evidence.
Appropriateness in light of regulatory guidance
In general, the five instruments considered for assessing the symptoms and impacts of GC demonstrated acceptable psychometric measurement properties. Some evidence of reliability and validity was available for each instrument, although the stand-alone FACT-G has not been formally evaluated in advanced GC.
The developers of these instruments did not document any information regarding data saturation, which is often required by regulatory agencies for justifying the content (both included and excluded) of items on an instrument. Additionally, while the developers designed their qualitative studies to include a sampling of countries, disease stages, and treatments, patients from a limited number of countries were included for all but the FACT-Ga, which conducted qualitative work in both North America and Asia. Prior to a regulatory submission, collaboration with the developers of the chosen instrument would be required to properly document both qualitative and quantitative evidence of the appropriateness of the instrument in an advanced GC population, with cognitive interviews in this population likely needed as a formal evidence of concept saturation.
Regulatory agencies, and particularly the US Food and Drug Administration, have expressed a preference for a 24-h recall period when posing PRO questions. The concern is that the response to an item that requires a longer period may involve calculations that differ across patients (e.g., how do patients average their experience over the past week?). In light of this, the MDASI-GI has an advantage of having a 24-h recall period, while the other four instruments refer to the past week or past seven days.
Value of PRO assessment in advanced GC clinical trials
A French phase-2 randomised controlled trial of 5-fluorouracil and leucovorin (LV5FU2) in combination with irinotecan versus LV5FU2 monotherapy or LV5FU2-cisplatin in 134 patients with metastatic GC adenocarcinoma found that after six months of treatment, patients in all the three groups experienced significant increases in the EORTC QLQ-C30 domains role, emotional, social, pain, insomnia, appetite loss, and global health. Furthermore, with the threshold of a ten-point or greater change in EORTC QLQ-C30 domain score as clinically significant, LV5FU2-irinotecan significantly improved on the role functioning, social functioning, and appetite loss domains; LV5FU2 monotherapy improved on the emotional functioning, nausea/vomiting, pain, insomnia, appetite loss, and constipation domains; and LV5FU2-cisplatin improved on the global health, physical functioning, role functioning, emotional functioning, fatigue, pain, insomnia, and appetite loss domains but worsened on dyspnea. LV5FU2 monotherapy was associated with poorer clinical outcomes and fewer QOL benefits. While the two LV5FU2 combination therapies improved a number of EORTC QLQ-C30 domains, LV5FU2-irinotecan was found to be superior to LV5FU2-cisplatin for PFS, and LV5FU2-cisplatin worsened dyspnea, which was not observed in LV5FU2-irinotecan [38].
Additionally, a large phase-3 randomised controlled trial comparing docetaxel and cisplatin plus fluorouracil (DCF) to cisplatin and fluorouracil (CF) in 445 patients with metastatic or locally recurrent GC or OGJ adenocarcinoma in 16 countries found that clinical outcomes favouring DCF were supported by PRO assessments. PROs, as assessed by the EORTC QLQ-C30 and the EQ-5D, showed that the time to global health deterioration was significantly longer for DCF. DCF also preserved EQ-5D VAS scores and EORTC QLQ-C30 scores related to physical functioning, social functioning, nausea/vomiting, pain, and appetite loss for a longer time versus CF, supporting the clinical superiority of DCF [39].
In a phase-3 randomised controlled trial of 333 European patients with metastatic or locally recurrent adenocarcinoma of the stomach or OGJ, the two treatments, irinotecan, folinic acid, and 5-fluorouracil (5-FU) (IF); and cisplatin and 5-FU (CF), were not differentiated by significant clinical outcomes (e.g., time to progression); however, scores on the EORTC QLQ-C30 and EQ-5D supported the superiority of IF with respect to PROs [40]. Between-group differences were significant for the EORTC QLQ-C30 and EQ-5D. Follow-up scores were significantly better in the IF arm as compared with the CF arm for EORTC QLQ C30 physical functioning (P = 0.003) and nausea/vomiting (P = 0.026) and for EQ-5D health state (P = 0.018) and VAS (P = 0.002).
Park et al [41] also reported the results of a clinical trial finding significant differences in treatment based on PRO assessments but not clinical endpoints. A single-centre phase-2 randomised controlled trial in Korea of paclitaxel plus 5-FU (PF) versus docetaxel plus 5-FU (DF) as first-line therapy for metastatic GC found that failure-free survival, OS, and ORR did not significantly differ between the two treatment arms. The EORTC QLQ-C30 found that both the arms showed clinically significant (ten-point change or greater) improvement in role, emotional, and constipation domains. Additionally, the PF arm was associated with clinically significant improvement in pain, dyspnea, and diarrhoea, and the DF arm with clinically significant improvement in cognitive functioning but clinically significant worsening in appetite.
Additional considerations for using PROs in advanced GC
When selecting an instrument for use in a population with advanced-stage cancer, the number of items in the instrument and its frequency of administration should be balanced to minimise patient burden and missing data. For example, completion rates for the EORTC QLQ-C30 in metastatic GC patients decreased over the course of treatment but were generally lower for patients who discontinued treatment and who experienced tumour progression [38]. In instruments developed for a more general population, administration is often as a daily diary, which may satisfy regulatory requirements of a short recall period. However, this type and frequency of administration are likely too burdensome for GC patients. Patients with the poorest QOL might be less likely to complete the PRO, which would lead to bias in the missing data. Furthermore, if PRO responses are solicited too frequently at home, the power provided by the additional data to be modelled longitudinally will likely be negated by the amount of missing patient responses. To balance the need for complete data as often as possible, one approach would be to administer PRO instruments at each study visit, prior to treatment. Feasibility studies also could be conducted to document the amount of effort required for completion.
One advantage held by the FACT-Ga is that it was developed concurrently in North America (Canada) and Asia (Japan). Because of the prevalence of GC in Asian populations, it is important to identify an appropriate instrument in terms of both the relevance of concepts across cultures and the suitability of translations. Good research practices in the linguistic translation and cultural adaptation of PRO instruments recommend forward and backward translation, reconciliation, harmonisation, patient cognitive interviews, and psychometric equivalency testing to ensure that the concepts measured by the instrument are relevant across the cultures and translations result in scores that mean the same thing regardless of language version [42]. Invariably, the PRO instruments used in Asian studies are linguistically translated from PRO instruments developed for implementation in Western countries. However, language translations might not be sufficient for cultural adaptation or for ensuring that the instrument’s concepts are important and clearly defined for an Asian population. With distal GC being more common in Asian populations and associated with symptoms of vomiting, heartburn, and indigestion, the EORTC QLQ-STO22 may be the most appropriate PRO instrument in this population, as it measures all the three of these symptoms.
Conclusions
As demonstrated by this targeted review of the literature in advanced-stage GC, there have been clinical studies in which significant or meaningful PRO differences were observed even in the absence of clinical differences. These findings suggest that PRO instruments may be sensitive to subtle treatment-related QOL or symptom benefits that cannot always be captured by common clinical instruments.
The EORTC QLQ-C30 along with the EORTC QLQ-STO22 module appears to provide the most comprehensive measurement of concepts important in GC with their extensive testing in GC patients, and this pair of instruments has the strongest body of psychometric evidence to support them. While the EORTC suite of instruments is the most widely used in advanced-stage GC, questionnaire length must be balanced against frequency of administration to minimise patient burden and missing data. Some of the brief PRO instruments developed for symptom assessment are administered as daily diaries, but this frequency of administration is likely too burdensome for GC patients. Patients with the poorest QOL and most severe symptoms would be the least likely to complete the instruments. Furthermore, the power afforded by the additional data to be modelled longitudinally as provided in daily diaries may be negated by the number of missing patient responses. For these reasons, the administration of PRO instruments at clinical study visits prior to treatment, rather than as daily diaries, is recommended. If the patient burden of the administration is of particular concern in a clinical study, the MDASI-GI could be considered in lieu of the EORTC QLQ-C30 and EORTC QLQ-STO22, as it includes fewer items and requires less administration time.
Future development of PRO instruments in advanced-stage GC should include topics of concern to patients without being overly burdensome due to length. Given the preliminary evidence that GC tumour anatomical location and, consequently, its symptomatology may be subjected to geographical variation, the analysis of efficacy and PRO data should include subgroup analysis by tumour location and geographic location in multicountry studies. In addition, given the prevalence of GC in Asian countries, there remains a concern that PRO instruments used in Asian studies have been linguistically translated, but not culturally adapted, from the instruments developed for use in Western countries and therefore do not measure all the elements of symptoms, symptom burden, and functional impacts in patients with GC.
GC symptoms and symptom burdens vary depending on a number of factors; however, this review supports the use of PROs, including evaluation of symptoms and symptom burdens, to show treatment benefit, even in the absence of clinical outcome differences. PRO instruments should be carefully selected for the study population and thoroughly translated, culturally adapted, and validated if being used in a language or culture other than the ones for which they were developed. Future development and validation efforts may be directed to shortening available scales to cover the concepts most important to patients with advanced-stage GC.
Conflict(s) of Interest
Professor Evans has received honoraria, consultancy fees, and support to attend conferences from Bristol Myers Squibb. He has also received honoraria and consultancy fees from Bayer, Roche, Otsuka, and Clovis and he has received research funding from many pharmaceutical companies.
Acknowledgments
The authors thank the researchers at Oxford Outcomes for conducting the literature review that served as a resource in preparing this article. Professor Evans is supported by Cancer Research UK and the Glasgow Experimental Cancer Medicine Centre (funded by Cancer Research UK and the Chief Scientist’s Office, Scotland). Drs. Coon and Copley-Merriman received financial support as consultants from Bristol-Myers Squibb.
References
1. Kelley JR and Duggan JM (2003) Gastric cancer epidemiology and risk factors J Clin Epidemiol 56 1–9 DOI: 10.1016/S0895-4356(02)00534-6 PMID: 12589864
2. Blot WJ et al (1991) Rising incidence of adenocarcinoma of the esophagus and gastric cardia JAMA 265 1287–9 DOI: 10.1001/jama.1991.03460100089030 PMID: 1995976
3. National Comprehensive Cancer Network (2012) Clinical practice guidelines in oncology: gastric cancer Version 2 Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site (registration required). Accessed August 23 2012
4. International Agency for Research on Cancer (2008) Cancer fact sheet: stomach cancer incidence, mortality and prevalence worldwide in 2008. http://globocan.iarc.fr/factsheet.asp. Accessed March 22 2012
5. Bowrey DJ et al (2006) Use of alarm symptoms to select dyspeptics for endoscopy causes patients with curable esophagogastric cancer to be overlooked Surg Endosc 20(11) 1725–8 DOI: 10.1007/s00464-005-0679-3 PMID: 17024539
6. Hosseini SN et al (2007) Delay in diagnosis and treatment of gastric cancer: from the beginning of symptoms to surgery—an Iranian study Turk J Gastroenterol 18(2) 77–81 PMID: 17602354
7. Maconi G, Manes G and Porro GB (2008) Role of symptoms in diagnosis and outcome of gastric cancer World J Gastroenterol 14(8) 1149–55 DOI: 10.3748/wjg.14.1149 PMID: 18300338 PMCID: 2690660
8. McLoughlin JM (2004) Adenocarcinoma of the stomach: a review Proc (Bayl Univ Med Cent) 17 391–9 PMCID: 1200678
9. Murad AM et al (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer Cancer 72 37–41 DOI: 10.1002/1097-0142(19930701)72:1<37::AID-CNCR2820720109>3.0.CO;2-P PMID: 8508427
10. Pyrhonen S et al (1995) Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer Br J Cancer 71 587–91 DOI: 10.1038/bjc.1995.114 PMID: 7533517 PMCID: 2033628
11. Glimelius B et al (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer Ann Oncol 8 163–8 DOI: 10.1023/A:1008243606668 PMID: 9093725
12. Webb A et al (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer J Clin Oncol 15 261–7 PMID: 8996151
13. van Cutsem E et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V25 study group J Clin Oncol 24(31) 4991–7 DOI: 10.1200/JCO.2006.06.8429 PMID: 17075117
14. Cunningham D et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer New Engl J Med 358 36–46 DOI: 10.1056/NEJMoa073149 PMID: 18172173
15. Bang YJ et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2- positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial Lancet 376 687–97 DOI: 10.1016/S0140-6736(10)61121-X PMID: 20728210
16. Bezjak A et al (2001) Oncologists’ use of quality of life information: results of a survey of Eastern Cooperative Oncology Group physicians Qual Life Res 10(1) 1–13 DOI: 10.1023/A:1016692804023 PMID: 11508471
17. Gondek K et al (2007) Current status of patient-reported outcomes in industry-sponsored oncology clinical trials and product labels J Clin Oncol 25(32) 5087–93 DOI: 10.1200/JCO.2007.11.3845 PMID: 17991926
18. Chau I et al (2004) Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer— pooled analysis from three multicenter, randomized, controlled trials using individual patient data J Clin Oncol 22(12) 2395–403 DOI: 10.1200/JCO.2004.08.154 PMID: 15197201
19. Bickenbach K and Strong VE (2012) Comparisons of gastric cancer treatments: East vs. West J Gastric Cancer 12(2) 55–62 DOI: 10.5230/jgc.2012.12.2.55 PMID: 22792517 PMCID: 3392325
20. Verdeccchia A et al (2003) Comparison of stomach incidence and survival in four continents Eur J Cancer 39 1603–9 DOI: 10.1016/S0959-8049(03)00360-5
21. Kim J et al (2010) Race and ethniciy correlate with survival in patients with gastric adenocarcinoma Ann Oncol 21 152–60 DOI: 10.1093/annonc/mdp290
22. Al-Refaie WB et al (2008) The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Data Base Cancer 113(3) 461–9 DOI: 10.1002/cncr.23572 PMID: 18553367
23. Hsu C et al (2012) Geographic difference in safety and efficacy of systemic chemotherapy for advanced gastric or gastroesophageal carcinoma: a met-analysis and meta-regression Gastric Cancer 15 265–80 DOI: 10.1007/s10120-011-0106-5 PMID: 22576708
24. Blazeby JM et al (2004) Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO22, to assess quality of life in patients with gastric cancer Eur J Cancer 40 2260–8 DOI: 10.1016/j.ejca.2004.05.023 PMID: 15454251
25. Garland SN et al (2011) Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy-gastric (FACT-Ga) quality-of-life instrument Cancer 117(6) 1302–12 DOI: 10.1002/cncr.25556
26. Wang XS et al (2010) Validation and application of a module of the M.D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI) Cancer 116 2053–63 DOI: 10.1002/cncr.24920 PMID: 20166216
27. Oñate-Ocaña LF et al (2009) Validation of the Mexican Spanish version of the EORTC C30 and STO22 questionnaires for the evaluation of health-related quality of life in patients with gastric cancer Ann Surg Oncol 16(1) 88–95 DOI: 10.1245/s10434-0080175-9
28. US Department of Health and Human Services, Food and Drug Administration (2009) Guidance for Industry: patient-reported outcome measures—use in medical product development to support labeling claims http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 20 March 2012
29. Vickery CW et al (2001) EORTC Quality of Life Group. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer Eur J Cancer 37(8) 966–71 DOI: 10.1016/S0959-8049(00)00417-2 PMID: 11334720
30. Eremenco SL, Cashy J and Webster K (2004) FACT-Gastric: a new international measure of QOL in gastric cancer. 2004 ASCO annual meeting proceedings (post-meeting edition) J Clin Oncol 22(14S, July 15 Suppl) 8123
31. Aaronson NK et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology J Natl Cancer Inst 85(5) 365–76 DOI: 10.1093/jnci/85.5.365 PMID: 8433390
32. Cella DF et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure J Clin Oncol 11(3) 570–9 PMID: 8445433
33. Morita S et al (2008) The domain structure of the EORTC QLQ-STO22 supported by Japanese validation data Psychooncology 17(5) 474–9 DOI: 10.1002/pon.1256
34. Sadighi S et al (2009) Quality of life in patients with gastric cancer: translation and psychometric evaluation of the Iranian version of EORTC QLQ-STO22 BMC Cancer 9 305 DOI: 10.1186/1471-2407-9-305 PMID: 19715606 PMCID: 2741487
35. European Organization for Research and Treatment of Cancer (EORTC). Validated modules by language. June 2011. http://groups.eortc.be/qol/eortc-modules Accessed 27 July 2011.
36. McDowell I (2006) Measuring Health: A Guide to Rating Scales and Questionnaires 3rd edn (New York: Oxford University Press)
37. MDAnderson.org. The MD Anderson Symptom Inventory (MDASI). 2012. http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/m-d-anderson-symptom-inventory.html. Accessed 13 August 2012
38. Bonnetain F et al (2005) Longitudinal quality of life study in patients with metastatic gastric cancer: analysis modalities and clinical applicability of QoL in randomized phase II trial in a digestive oncology Gastroenterol Clin Biol 29(11) 1113–24 DOI: 10.1016/S0399-8320(05)82175-X
39. Ajani JA et al (2007) Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group J Clin Oncol 25(22) 3210–16 DOI: 10.1200/JCO.2006.08.3956 PMID: 17664468
40. Curran D et al (2009) Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: results of a randomised phase III trial Qual Life Res 18(7) 853–61 DOI: 10.1007/s11136-009-9493-z PMID: 19568958 PMCID: 2724642
41. Park SH et al (2006) Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil Anticancer Drugs 17 225–9 DOI: 10.1097/00001813-200602000-00015 PMID: 16428942
42. Wild D et al (2005) Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation Value Health 8 94–104 DOI: 10.1111/j.1524-4733.2005.04054.x PMID: 15804318






