Chemotherapy and anti-HER2 therapy in metastatic breast cancer in pregnancy followed by surgical treatment
Julia Berwart1 and Fedro A Peccatori2
1Department of Gynaecology, Clemente Alvarez Emergency Hospital, Rosario, S2002QEA Argentina
2Fertility and Procreation Unit, European Institute of Oncology, IRCCS, 20141 Milan, Italy
Abstract
Between 5% and 10% of women have distant metastases when they receive a Breast cancer (BC) diagnosis. Metastatic BC is associated with poor prognosis but advances in systemic treatments have improved survival rates in recent decades.
Debates about local primary tumour management in metastatic stages continue, but multiple studies have shown that primary tumour surgery can be beneficial.
BC is one of the most commonly diagnosed neoplasias during pregnancy. Treatment of pregnant BC patients should follow the standard treatment of young, non-pregnant patients as closely as possible.
We present the case of a young, pregnant patient with metastatic BC with a complete clinical response to chemotherapy followed by surgical treatment.
Keywords: Breast cancer, pregnancy, stage IV, surgical treatment, primary tumour
Correspondence to: Julia Berwart
Email: dra.juliaberwart@gmail.com
Published: 14/05/2019
Received: 18/12/2018
Publication costs for this article were supported by the ecancer Global Foundation.
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women of childbearing age [1].
Between 5% and 10% of women have distant metastases when they receive a BC diagnosis [2].
Diagnosis of BC in pregnancy (BCP) is uncommon, representing 1%–5% of all diagnoses in young women, with an incidence of 1:3,000 pregnancies [3].
BCP normally appears at later stages than BC in non-pregnant patients, often due to delayed diagnosis [4].
We present the case of a young, pregnant patient with metastatic BC with a complete clinical response to chemotherapy followed by surgical treatment. We then present a bibliographic review.
Case report
In 2018, a 33-year-old patient, Gravida 3, Caesarean 2, was evaluated in the 16th week of pregnancy. During the physical examination, tumour was palpated in the retroareolar region. This tumour was of increased consistency, with a maximum diameter of approximately 10 cm, irregular margins and clinically negative axilla. Needle biopsy: infiltrating ductal carcinoma, histological grade 3. Immunohistochemistry results: oestrogen receptor-positive (35%), progesterone receptor-positive (85%), HER2/neu-positive (Score 3+), Ki67 = 37%. Whole-body nuclear magnetic resonance without contrast found images consistent with hepatic metastasis of segment V measuring 29 mm (Figure 1) and millimetric metastases in the right iliac bone. Genetic test was negative (BRCA 1 and 2 not mutated).
The patient received chemotherapy after cardiological assessment with electrocardiogram (epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2) for four cycles during pregnancy. Treatment led to partial Breast tumour remission and complete response of the lesion in the iliac region but the hepatic lesion increased in size. Dosages were calculated based on body surface area using patient weight at chemotherapy. The last cycle was administered 5 weeks before delivery to avoid maternal and child toxicity at birth.
This tumour was HER2-positive but anti-HER2 therapy is contraindicated during pregnancy. This case was thus discussed in a meeting of a multidisciplinary team, which decided to anticipate delivery. At 35 weeks and 4 days, 4 weeks after the last chemotherapy cycle, a healthy child was born weighing 2,345 g and measuring 49 cm. The patient had a scheduled caesarean section due to her obstetric history of two prior caesarean births. Pathological examination of the placenta was negative.
The patient continued treatment with trastuzumab and docetaxel for eight cycles. Whole-body nuclear magnetic resonance without contrast after chemotherapy ended found: Breast lesion reduced by more than 50% and the size and functionality of the hepatic lesion reduced. The patient continued treatment with trastuzumab + pertuzumab, Decapeptyl and exemestane. Whole-body nuclear magnetic resonance 1 year later found: complete remission of hepatic (Figure 2) and bone lesions. Breast ultrasound found two millimetric formations measuring 10 and 7 mm in the upper outer quadrant of the right Breast.
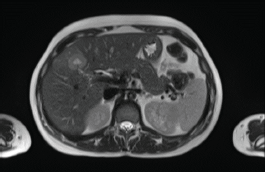
Figure 1. Whole-body nuclear magnetic resonance without contrast: images consistent with hepatic metastasis of segment V measuring 29 mm.
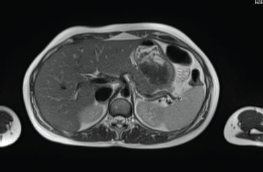
Figure 2. Whole-body nuclear magnetic resonance without contrast: complete remission of the hepatic lesion.
The multidisciplinary team and the patient met to discuss surgery. The patient then had a mastectomy with nipple-areola complex preservation, placement of a definitive Breast prosthesis and contralateral mastopexy. A biopsy of sentinel lymph nodes was negative. Pathological examination found lobular in situ neoplasia (LIN2) associated with fibrosis.
The patient enjoys good health 22 months after diagnosis, is in excellent condition and free of disease, with a normal echocardiogram.
Discussion
Metastatic BC has historically been considered incurable [5]. Primary therapy is systemic (chemotherapy, hormonal and biological treatment) [6, 7] and aims to reduce disease progression and improve quality of life.
Metastatic BC is associated with a poor prognosis but advances in systemic treatments have improved survival rates in recent decades [8–11].
BC is one of the most commonly diagnosed neoplasias during pregnancy [12]. Termination is generally not necessary and pregnancy does not worsen BC prognosis. However, patients with an unfavourable prognosis and advanced-stage disease in the first trimester should consider termination of the pregnancy [13].
Staging during pregnancy must consider the risks of ionising radiation, and bone scintigraphy and computed axial tomography are thus contraindicated. It is possible to use whole-body nuclear magnetic resonance without contrast in pregnant patients with locally advanced tumours [14].
Treatment of BCP should follow the standard treatment of young, non-pregnant patients as closely as possible.
Most treatments can be used safely, depending on the gestational age.
Chemotherapy is contraindicated during the first trimester of pregnancy due to its association with foetal malformations. It is recommended that, whenever possible, the second and third trimesters treatment follow guidelines for treating young, non-pregnant patients [15]. Various drugs (doxorubicin, epirubicin, docetaxel and paclitaxel) have shown a constant decrease in the plasma concentration curve and increased clearance during pregnancy. These changes can affect levels of free drug in the blood, which can reduce therapeutic effectiveness. However, no study has shown reduced therapeutic effectiveness or lower survival compared to non-pregnant patients [16]. Doses should not be modified during pregnancy and should be calculated based on actual patient weight. Pregnant patients should receive the last chemotherapy dose at least 4 weeks before birth to avoid administering high drug concentrations to infants at birth [17].
The same adjuvant or neoadjuvant treatment that non-pregnant patients receive, with anthracyclines and taxanes, is recommended after the first trimester. The recommended regimen is anthracyclines followed by taxanes. Epirubicin has better placental passage than doxorubicin and is, therefore, the treatment of choice [13]. The use of weekly paclitaxel is also preferable to docetaxel due to the lower haematological toxicity of the former [18].
Some agents, such as anti-HER2 drugs and endocrine therapy are contraindicated as they can cause foetal toxicity. Trastuzumab is associated with oligo-anhydramnios when administered for long periods in the second and third trimesters [19].
One of the most crucial factors that determine foetal health is gestational age at delivery. Trastuzumab should, therefore, be avoided before 37 weeks, but each case requires a personalised discussion. Our patient had a lack of hepatic response and could receive anti-HER2 treatment after pregnancy. Based on those factors, her pregnancy was ended at 35 weeks, after full foetal pulmonary development.
Breastfeeding is generally not recommended during chemotherapy as drugs are excreted in human milk. Excretion depends on the capacity of the drug to bind to plasma proteins, as well as its ionisation and liposolubility [20].
Breastfeeding becomes possible 3 weeks after chemotherapy ends. One study compared women who underwent chemotherapy during pregnancy with non-exposed women. That study found a significant difference in patient perception of reduced milk supply and a greater need to supplement the diets of their infants [21].
Surgery in stage IV has historically been limited to palliative treatment of primary tumour complications, such as bleeding, ulceration and infections at the tumour site (surgery described as a ‘toilet’ mastectomy); or for complications at other sites (spinal cord compression, pathological bone fractures, resection of single bone or brain metastases and pleurodesis). Debates about the impact of stage IV primary tumour surgery on survival continue.
It has been shown that controlling other types of primary tumours, such as metastatic renal, colorectal and gastric cancers and melanomas, can improve survival [22–25]. Removing the primary tumour may have an immunomodulating effect, reducing tumour load, eliminating a possible source of metastasis or reducing the likelihood that potentially resistant cells will develop [26, 27].
It has also been suggested that surgically resecting the primary tumour can stimulate the growth of metastases and shorten survival [28, 29]. Other hypotheses assume that BC spreads via blood and the lymphatic vessels. Local control is thus not important as it does not affect the development of subsequent metastases [30].
Multiple retrospective studies suggest that primary tumour surgery in patients with stage IV BC can be beneficial [31–34].
One meta-analysis evaluated ten retrospective studies with a total of 28,693 patients. That study suggested that survival times improved in selected patients who underwent primary tumour surgery (40%) compared to patients who only received systemic therapy (22%) [35]. This improvement in survival may reflect selection bias: younger, healthier patients with low tumour loads, oligometastasis or metastasis to a more favourable site and tumours with better biological profiles [36].
There are six randomised and controlled studies underway in various countries: the United States, Austria, the Netherlands, Japan, India and Turkey. We now have results from two of these studies.
The first is an Indian study that selected 350 patients with stage IV BC who responded to initial systemic therapy. These patients were randomised into two groups, one that received local-regional treatment (LRT) and another group that did not. This study found no differences in overall 2-year survival between the two groups: 43% in the non-LRT group and 42.9% in the LRT group [37].
The second study was conducted in Turkey and randomised 278 patients: one group received only systemic therapy and the other received LRT followed by systemic therapy. This study found a higher 5-year overall survival rate for the second group, 41.6%, versus 24% for the first group. This study also found that the patients who survived longer were those under age 55 with positive hormone receptors, HER2-negative disease and single bone metastases [38].
Conclusion
Metastatic BC can be treated effectively during pregnancy. Anthracycline and taxane chemotherapy administered during pregnancy is effective for patients and safe for foetuses. Conversely, trastuzumab, pertuzumab and endocrine therapy are contraindicated as they can cause foetal harm.
LRT merits strong consideration in patients like ours, who have the good general condition, systemic oligometastatic disease and favourable tumour biology. However, clinicians must also consider the age of the patient. A multidisciplinary team which includes oncologists, Breast specialists, obstetricians, paediatricians, surgeons, psychologists and nurses trained to provide comprehensive care is vital.
References
1. Rosenberg SM, Newman LA, and Partridge AH (2015) Breast cancer in young women: rare disease or public health problem? JAMA Oncol 1 877–878 https://doi.org/10.1001/jamaoncol.2015.2112 PMID: 26204453
2. Howlader N, Noone AM, and Krapcho M, et al (1975–2008) SEER cancer statistics review Bethesda: National Cancer Institute [http://seer.cancer.gov/csr/1975_2008/]
3. Pereg D, Koren G, and Lishner M (2008) Cancer in pregnancy: gaps, challanges and solutions Cancer Treat Rev 34 302–312 https://doi.org/10.1016/j.ctrv.2008.01.002 PMID: 18291591
4. Amant F, von Minckwitz G, and Han SN, et al (2013) Prognosis of woman with primary Breast cancer diagnosed during pregnancy: results from an international collaborative study J Clín Oncol 31(20) 2532–2539 https://doi.org/10.1200/JCO.2012.45.6335
5. Wood WC, Muss HB, and Solin LJ, et al (2005) Cancer of the Breast Cancer Principles and Practice of Oncology eds VT Jr DeVita, S Hellman, and SA Rosenberg (Philadelphia, PA: Lippincott Williams and Wilkins) pp 1453–1462
6. Bernard-Marty C, Cardoso F, and Piccart MJ (2004) Facts and controversies in systemic treatment of metastatic Breast cancer Oncologist 9 617–632 https://doi.org/10.1634/theoncologist.9-6-617 PMID: 15561806
7. Hortobagyi GN (1998) Treatment of Breast cancer N Engl J Med 339 974–984 https://doi.org/10.1056/NEJM199810013391407 PMID: 9753714
8. Fangjian G, Yong-fang K, and Ya Chen T, et al (2018) Trends in Breast cancer mortality by stage at diagnosis among young women in the United States Cancer 124(17) 3500–3509 https://doi.org/10.1002/cncr.31638
9. Hortobagyi GN (1998) Treatment of Breast cancer N Eng J Med 339 974–984 https://doi.org/10.1056/NEJM199810013391407
10. Giordano SH, Buzdar AU, and Smith TL, et al (2004) Is Breast cancer survival improving? Cancer 100 44–52 https://doi.org/10.1002/cncr.11859
11. Andre F, Slimane K, and Bachelot T, et al (2004) Breast cancer with synchronous metastases: trends in survival during a 14-year period J Clin Oncol 22 3302–3308 https://doi.org/10.1200/JCO.2004.08.095 PMID: 15310773
12. Lee YY, Roberts CL, and Dobbins T, et al (2012) Incidence and outcomes of pregnancy-associated cancer in Australia, 1994-2008: a population-based linkage study BJOG 119(13) 1572–1582 https://doi.org/10.1111/j.1471-0528.2012.03475.x PMID: 22947229 PMCID: 3533794
13. Peccatori FA, Azim Jr HA, and Scarfone G, et al (2009) Weekly epirubicin in the treatment of gestational Breast cancer (GBC) Breast Cancer Res Treat 115 591–594 https://doi.org/10.1007/s10549-008-0159-2
14. Peccatori FA, Codacci-Pisanelli G, and Del Grande M, et al (2017) Whole body MRI for systemic staging of Breast cancer in pregnant women Breast 35 177–181 https://doi.org/10.1016/j.breast.2017.07.014 PMID: 28756339
15. Peccatori FA, Azim Jr HA, and Orecchia R, et al (2013) Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up Ann Oncol 24(6) vi160–vi170 https://doi.org/10.1093/annonc/mdt199 PMID: 23813932
16. Amant, F, Deckers S, and Van Calsteren K, et al (2010) Breast cancer in pregnancy: recommendations of an international consensus meeting Eur J Cancer 46(18) 3158–3168 https://doi.org/10.1016/j.ejca.2010.09.010 PMID: 20932740
17. Van Hasselt JG, Van Calsteren K, and Heyns L, et al (2014) Optimizing anticancer drug treatment in pregnant cancer patients: pharmacokinetic analysis of gestation-induced changes for doxorubicin, epirubicin, docetaxel and paclitaxel Ann Oncol 25 2059–2065 https://doi.org/10.1093/annonc/mdu140 PMID: 24713311
18. Loibl S, Schmidt A, and Gentilini O, et al (2015) Breast cancer diagnosed during pregnancy—adapting recent advances in Breast cancer care for pregnant patients JAMA Oncol 1(8) 1145–1153 https://doi.org/10.1001/jamaoncol.2015.2413 PMID: 26247818
19. Zagouri F, Sergentanis TN, and Chrysikos D, et al (2013) Trastuzumab administration during pregnancy: a systematic review and meta-analysis Breast Cancer Res Treat 137(2) 349–357 https://doi.org/10.1007/s10549-012-2368-y
20. Ngu SF and Ngan HY (2016) Chemotherapy in pregnancy Best Pract Res Clin Obstet Gynaecol 33 86–101 https://doi.org/10.1016/j.bpobgyn.2015.10.007
21. Stopenski S, Aslam A, and Zhang X, et al (2017) After chemotherapy treatment for maternal cancer during pregnancy, is Breastfeeding possible? Breastfeed Med 12 91–97 https://doi.org/10.1089/bfm.2016.0166 PMID: 28170295
22. Flanigan RC, Salmon SE, and Blumenstein BA, et al (2001) Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer N Engl J Med 345 1655–1659 https://doi.org/10.1056/NEJMoa003013
23. Essner R, Lee JH, and Wanek LA, et al (2004) Contemporary surgical treatment of advanced-stage melanoma Arch Surg 139(9) 961–966 https://doi.org/10.1001/archsurg.139.9.961 PMID: 15381613
24. Rosen SA, Buell JF, and Yoshida A, et al (2000) Initial presentation with stage IV colorectal cancer: how aggressive should we be? Arch Surg 135(5) 530–534 https://doi.org/10.1001/archsurg.135.5.530 PMID: 10807276
25. Hallissey MT, Allum WH, and Roginski C, et al (1988) Palliative surgery for gastric cancer Cancer 62(2) 440–444 https://doi.org/10.1002/1097-0142(19880715)62:2<440::AID-CNCR2820620232>3.0.CO;2-N PMID: 2454725
26. Danna EA, Sinha P, and Gilbert M, et al (2004) Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease Cancer Res 64(6) 2205–2211 https://doi.org/10.1158/0008-5472.CAN-03-2646 PMID: 15026364
27. Norton L and Massague J (2006) Is cancer a disease of self-seeding? Nat Med 12(8) 875–878 https://doi.org/10.1038/nm0806-875 PMID: 16892025
28. Demicheli R, Valagussa P, and Bonadonna G (2001) Does surgery modify growth kinetics of Breast cancer micrometastases? Br J Cancer 85 490–492 https://doi.org/10.1054/bjoc.2001.1969 PMID: 11506484 PMCID: 2364103
29. Retsky M, Bonadonna G, and Demicheli R, et al (2004) Hypothesis: induced angiogenesis after surgery in premenopausal node-positive Breast cancer patients is a major underlying reason why adjuvant chemotherapy works particularly well for those patients Breast Cancer Res 6 R372–R374 https://doi.org/10.1186/bcr804 PMID: 15217504 PMCID: 468653
30. Fisher B (1992) The evolution of paradigms for the management of Breast cancer: a personal perspective Cancer Res 52 2371–2383 PMID: 1568206
31. Carmichael AR, Anderson EDC, and Chetty U, et al (2003) Does local surgery have a role in the management of stage IV Breast cancer? Eur J Surg Oncol 29 17–19 https://doi.org/10.1053/ejso.2002.1339 PMID: 12559070
32. Gnerlich J, Jeffe DB, and Deshpande AD, et al (2007) Surgical removal of the primary tumor increases overall survival in patients with metastatic Breast cancer: analysis of the 1988–2003 SEER data Ann Surg Oncol 14 2187–2194 https://doi.org/10.1245/s10434-007-9438-0 PMID: 17522944
33. Blanchard DK, Bhatia P, and Hilsenbeck SG, et al (2006) Does surgical management of stage IV Breast cancer affect outcome? Breast Cancer Res Treat 2006(1) 100–118
34. Khan SA, Stewart AK, and Morrow M (2002) Does aggressive local therapy improve survival in metastatic Breast cancer? Surgery 132 620–627 https://doi.org/10.1067/msy.2002.127544 PMID: 12407345
35. Harris E, Barry M, and Kell MR (2013) Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV Breast cancer impacts on survival Ann Surg Oncol 20 2828–2834 https://doi.org/10.1245/s10434-013-2998-2 PMID: 23653043
36. Khan SA (2007) Primary tumor resection in stage IV Breast cancer: consistent benefit, or consistent bias? Ann Surg Oncol 14(12) 3285–3287 https://doi.org/10.1245/s10434-007-9547-9 PMID: 17891444 PMCID: 2077920
37. Badwe R, Hawaldar R, and Nair N, et al (2015) Locoregional treatment versus no treatment of the primary tumour in metastatic Breast cancer: an open-label randomised controlled trial Lancet Oncol 16 1380–1388
38. Soran A, Ozmen V, and Ozbas S, et al (2018) Randomized trial comparing resection of primary tumor with no surgery in stage IV Breast cancer at presentation: protocol MF07-01 Ann Surg Oncol 25 3141 https://doi.org/10.1245/s10434-018-6494-6 PMID: 29777404






