Anti-androgenic effects of flavonols in prostate cancer
Tristan Boam
Royal Liverpool University Hospital, Prescot Street, Liverpool, Merseyside, L7 8XP, UK
Correspondence to: Tristan Boam. Email: Tristanboam@hotmail.com
Abstract
Dietary-derived agents, such as the flavonoids, are of particular interest for prostate cancer (PCa) chemoprevention as they may offer a favourable safety and side-effect profile. An agent that demonstrates action on the androgen receptor (AR) axis may have value for preventing or treating castrate-resistant PCa. Four main flavonols – quercetin, myricetin, kaempferol, and fisetin – have been demonstrated in laboratory studies to have chemopreventive action in both castrate-resistant and castrate-sensitive PCa models. Mechanisms of flavonol action on the AR axis in PCa have been proposed to be inhibition of the 5α-reductase enzymes, direct androgen competition, suppression of the AR complex and transactivation by coregulators such as c-Jun, Sp1, and the PI3K/Akt pathway. It is, however, still unclear with current levels of evidence whether AR axis-mediated effects can fully account for the flavonols’ chemopreventive action.
Keywords: androgen, chemoprevention, fisetin, flavonoids, flavonols, kaempferol, myricetin, prostate cancer, quercetin
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 22/10/2015; Received: 09/06/2015
Introduction
Prostate cancer (PCa) is a leading cause of morbidity and mortality within the male population. It was the cancer with the highest incidence rate in men in the United Kingdom in 2013, a total of 40,372 new cases were diagnosed, accounting for 27% of all new male cancer diagnoses [1]. There is increasing evidence that a western diet along with other environmental influences may be a significant risk factor for the development of PCa. A lower incidence is observed in vegetarians and residents of Japan and China where diets are composed of more fruits, vegetables and soy products rather than processed and high glycaemic index foods [2, 3]. Interestingly, migrants from Asian countries eventually develop a PCa risk approaching that of their western counterparts, suggesting that diet and lifestyle factors have a significant role to play in the pathogenesis of PCa [4–9]. Recently, interest has turned towards identifying dietary components that may exert an anti-carcinogenic effect on PCa. Early phase-I and phase-II clinical trial results of dietary-derived agents, such as lycopene, curcumin, and isoflavone, show that they are well tolerated and have favourable effects on PCa activity markers, such as prostate-specific antigen (PSA) and serum testosterone levels [10, 11]; however, further evidence is needed to determine their value for the prevention or treatment of PCa.
The flavonols, a class of phenolic phytochemicals exemplified by quercetin, myricetin, kaempferol, and fisetin (Figure 1) can be found in foods, such as olives, onions, kale, and cranberries [12]. They have been shown to arrest PCa growth, invasion, and metastasis in laboratory studies in vitro and in vivo [13–20]. A US-led project investigating the potential use of fisetin to treat androgen-dependent PCa is currently in its pre-clinical stages. (US Appl No: 12/412945, 2009)
Their structural similarity to androgens (testosterone is shown) is evident, and hence, AR receptor interaction has been postulated as a mechanism for their anti-carcinogenic activity in PCa.
Development of PCa is thought to be a multistep process from the normal prostate epithelium to intraepithelial neoplasia (benign prostatic hyperplasia is not currently considered as a precursor to PCa), then localised carcinoma before progression to invasive or metastatic disease. Treatment options for advanced or metastatic disease are limited to androgen ablation and chemotherapy. Initially, most cancers are responsive to this; however, after a period of time, recurrences are common as the disease progresses to a castrate-resistant phenotype (CRPCa), for which there is a very poor prognosis [21]. Transition to CRPCa is likely due to changes within the androgen-receptor (AR) signalling axis and so this mechanism has become an attractive target for research into novel therapies [22].
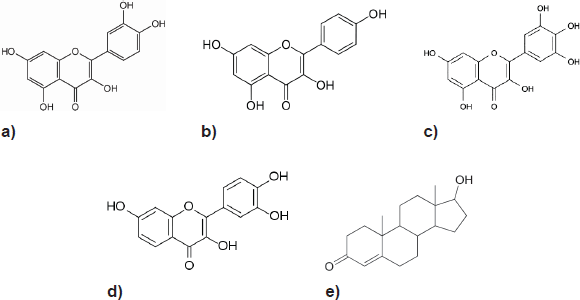
Figure 1. Structure of the four main flavonols: (a) Quercetin, (b) Kaempferol, (c) Myricetin, (d) Fisetin and (e) Testosterone.
ARs are present in most human tissues; however, they are most important for driving the development of sexual characteristics. The AR-signalling pathway is essential for maintaining normal metabolic function, cell proliferation, and homeostasis [23] and is an important component in the early pathogenesis of PCa. Regardless of whether prostate tissue is normal or neoplastic, its growth and survival is still dependant on AR signalling. The difference lies in the fact that CRPCa does not require an external stimulus of androgens. Many potential reasons for this exist, the most common stemming from AR mutations and aberrant post-translational modifications, causing the system to become constituently active even in the absence of external androgens. AR overexpression leading to hypersensitivity, activation by coregulators, non-androgenic ligands, and alternative pathways may also play a role [24].
The AR axis has previously been a target for chemopreventive agents in PCa. The 5α-reductase inhibitors finasteride [25, 26] and dutasteride [27] were the subject of clinical trials that seem to show a benefit of these agents for PCa chemoprevention. The systemic reduction in dihydrotestosterone (DHT) levels associated with an apparent reduction in PCa incidence in the trial participants may represent an AR axis-mediated role for chemoprevention. The 5α-reductase inhibitors, however, unfortunately have sexual side-effects and are currently still not licensed for PCa chemoprevention.
Androgen competition
Flavonols are related structurally to oestrogens and this similarity has led to the hypothesis that they exert their action either by competing with androgens for AR binding sites or by affecting endogenous androgen levels.
Myricetin, quercetin, and fisetin have a catechol or pyrogallo configuration in the B-ring which may allow them to inhibit 5α-reductase isoenzyme 1, while kaempferol has been shown to inhibit isoenzyme 2 in transfected Rat 1A cells [28]. This may suggest that regular inclusion of these compounds in the diet may reduce DHT levels and consequently the androgenic stimulated growth of PCa. Kaempferol has also been shown to inhibit DHT stimulated cell growth in LNCaP cells and in contrast to the previous study, reduce expression of the 5α-reductase isoenzyme 1 gene [29]. This discrepancy may have arisen because of the difference in cell lines used but nevertheless supports the hypothesis that the flavonols have some activity on the 5α-reductases. It is still unclear, however, if a possible reduction in DHT levels would contribute a major androgen-dependant effect on their chemopreventive action.
Quercetin has been shown to compete with testosterone at high concentrations. However, the concentrations are higher than needed for cell growth inhibition, suggesting that this is unlikely to be its main cytostatic mechanism [30]. Another study found that quercetin increases serum testosterone, and after an initial rise, decreases DHT in a dose-dependent manner in a rat model. The rat prostates were found to have dilated lumens full of secretory materials. When coadministered with finasteride, it was shown to reduce rat prostate weight and negate the DHT-lowering effect of finasteride. The group hypothesised that quercetin acted synergistically with finasteride through an androgen-independent pathway to reduce the prostate weight. Quercetin may therefore hold value in combination to reduce the dose and therefore adverse effects of finasteride in benign prostatic hyperplasia or patients with PCa [31]. The results of these studies do not support quercetin as acting through androgen level modulation and displacement as its main chemopreventive action.
Khan et al observed that fisetin suppresses the growth of CWR22rv1 and LNCaP cells in vitro and competes with a labelled high-affinity androgen for the ligand-binding domain of the AR and in addition inhibits DHT-driven stabilisation of AR, leaving it vulnerable to proteasome degradation [32]. This would point to an AR-mediated effect being a large component of fisetin’s mechanism of action in vitro; however, there is a lack of other studies to support this hypothesis. It is also possible that fisetin was acting through a separate pathway, and the effects on the AR pathway are a consequence, rather than a cause of this action.
Androgen receptor expression and activity
Many studies have focused on the effect of flavonols on the expression of the AR itself and its activity in the form of AR-dependant genes. AR luciferase reporter genes have been used to demonstrate that quercetin [33] and Kaempferol compounds [34] antagonise AR activity in the LNCaP cell line.
In terms of AR expression itself, quercetin seems to have varying effects. A reduction in AR expression in quercetin-treated CWR22Rv1 cells was observed in serum-free cultures, yet an increase in expression was seen when 10% foetal bovine serum was added [35]. In the LNCaP cell line, quercetin inhibited AR protein expression (36). Quercetin has also been shown to activate the mutant AR T877A (a variant able to respond to multiple ligands); however, in the same study, it increased overall AR expression while suppressing AR mRNA levels [37].
The AR-dependant genes PSA, 5α-reductase and human kallikrein 2 (KLK2) serve as markers of AR activity. Quercetin was shown to suppress the gene expression of PSA and KLK2 at the transcriptional level [36, 38], while kaempferol exhibited the same activity with the PSA and 5α-reductase genes, also interestingly inhibiting AR accumulation in the nucleus [29]. In one study, however, quercetin treatment was shown to increase PSA levels [37]. High PSA levels are associated with a worse prognosis in PCa and it is unclear whether a reduction in levels by flavonols would have a beneficial effect in itself or whether it serves as a marker of chemopreventive action. Khan et al were able to demonstrate a less ambiguous effect of fisetin in their study. In the CWR22rv1 and LNCaP cell lines, treatment with fisetin suppressed AR expression, inhibited protein and mRNA expression of AR target genes, and reduced PSA levels in a mouse xenograft (32).
These studies show that there is likely to be a suppressive effect of flavonols on the AR expression and activity. They are able to achieve this in the androgen-dependant LNCaP cell line and the more insensitive CW22Rv1 line. This may be a sign that flavonols have potential for the prevention or treatment of CRPCa via action on the AR axis, independent of direct ligand-receptor stimulation.
Androgen receptor transactivators
Yuan et al have looked extensively into the activity of quercetin on AR transactivation by coregulators, hypothesising that these may the main mechanism of action of its effects on the AR rather than a direct interaction. An increased expression of the coregulator c-Jun was observed in LNCaP and LAPC4 PCa cells treated with quercetin. It is thought that c-Jun works to inhibit AR expression, and in this study, an inhibition of AR at the transcriptional level was observed [39]. Sp1 is another coregulator that has a promotional effect on the AR, quercetin was shown to attenuate this effect possibly by increasing Sp1-binding affinity for the C-terminal domain of the AR. Androgen-dependant post-translational phosphorylation of the AR was also reduced by quercetin [38], suggesting a blunting of its action. A later study suggested that quercetin achieves its anti-androgenic effect by promoting a c-Jun-Sp1-AR complex that reduced overall AR activity [40].
Aalinkeel et al were able to demonstrate that quercetin induces apoptosis in the largely androgen-independent PC-3 and DU145 PCa cell lines as a direct result of their inhibition of heat shock protein 90 [15], a key regulator of AR nuclear translocation [22, 41]. Whether this implies that an AR-mediated effect was contributory to the apoptotic process remains unclear.
The phosphatidylinositol 3-kinase/Akt (PI3K/Akt)-signalling axis serves as an AR transactivator, with Akt-dependant phosphorylation of the AR increasing its transcriptional activity [24, 42]. Flavonols have been shown in multiple studies to suppress Akt in conjunction with a growth-inhibitory effect on PCa cells [18, 19, 43–45]. A quercetin analogue-based PI3K inhibitor was developed as a PCa-specific prodrugactivated by PSA cleavage [46]. Inhibition of PI3K in this manner may suppress Akt-driven transactivation of AR, and hence, this drug may have a more potent anti-androgenic effect than unmodified quercetin.
Conclusions
The hormone-refractory nature of advanced PCa has meant that the AR has been a target of much research, especially into dietary-derived potential chemopreventive agents, such as the flavonols. Quercetin has been the most extensively studied of this group, with evidence lacking as to the other compounds’ effect on the AR axis.
Whether or not flavonols exert their main effect through androgen competition remains ambiguous. All four main flavonols seem to have an inhibitory action on the 5α-reductases, hypothetically reducing DHT levels and blunting androgenic stimulation of growth in PCa. It is unclear, however, whether this would translate into a clinically relevant effect that is similar to finasteride and dutasteride. There is little evidence to show that flavonols directly compete with androgen binding to the AR. Fisetin demonstrated this ability in one study whilst quercetin was only able to compete at concentrations higher than needed to achieve its cytostatic effect. As the flavonols seem to exhibit an AR-mediated effect on the largely androgen-independent cell lines CW22Rv1 and PC-3 [14] without convincing evidence that they compete directly with androgens, it is reasonable to assume that they exert their action on the AR through a separate pathway.
While it is undeniable that these flavonols exhibit action on the AR, it remains unclear, and in some places unlikely that their main effect is AR axis mediated. Indeed, flavonols have shown efficacy in many cancer types, such as lung and colon, that are not reliant on androgenic stimuli for progression [47, 48]. It is possible that flavonols may act via AR transactivators. Quercetin, kaempferol and fisetin have all been shown to reduce expression of the AR itself as well as its transcriptional activity on AR-inducible genes. Yuan et al hypothesised that quercetin’s action was mediated through the co-regulators c-Jun and Sp1; however, it remains to be seen whether kaempferol and fisetin act in a similar way.
If the AR-signalling pathway is indeed the key to preventing or treating CRPCa, then methods to enhance flavonols’ effect on it may be beneficial. Structural modification of the flavonols, for example the quercetin-based PI3K inhibitor mentioned previously, is a potential avenue for increasing their action on the AR pathway. Another potential solution is using them in combination with other agents, for example other flavonoids [35, 45, 49, 50] or more well-established anti-androgenic agents, such as finasteride [31] and tamoxifen [51] which have demonstrated efficacy in tandem with quercetin in animal models of PCa.
In conclusion, flavonols exhibit a modest effect on the AR-signalling axis, but their value as chemopreventives or adjuncts to existing or novel treatments for CRPCa is promising.
Conflict of interest
The author declares that they have no conflict of interest.
List of abbreviations
PCa Prostate cancer
CRPCa Castrate-resistant prostate cancer
AR Androgen receptor
DHT Dihydrotestosterone
PSA Prostate specific antigen
KLK2 Human kallikrein 2
References
1. Adapted from data from the office for national statistics licensed under the open government licence v.1.0 Cancer registrations in England 2013. 2015 Available at: http://www.ons.gov.uk/ons/search/index.html?newquery=prostate Accessed 09/20, 2015
2. Hori S, Butler E and McLoughlin J (2011) Prostate cancer and diet: food for thought? BJU Int 107(9) 1348–1359 DOI: 10.1111/j.1464-410X.2010.09897.x PMID: 21518228
3. Hardin J, Cheng I and Witte JS (2011) Impact of consumption of vegetable, fruit, grain, and high glycemic index foods on aggressive prostate cancer risk Nutr Cancer 63(6) 860–872 DOI: 10.1080/01635581.2011.582224 PMID: 21774611 PMCID: 3209415
4. Shimizu H, Ross RK and Bernstein L et al (1991) Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles county Br J Cancer 63(6) 963–966 DOI: 10.1038/bjc.1991.210 PMID: 2069852 PMCID: 1972548
5. Cook LS, Goldoft M and Schwartz SM et al (1999) Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants J Urol 161(1) 152–155 DOI: 10.1016/S0022-5347(01)62086-X PMID: 10037388
6. Luo W, Birkett NJ and Ugnat AM et al (2004) Cancer incidence patterns among Chinese immigrant populations in Alberta J Immigr Health 6(1) 41–48 DOI: 10.1023/B:JOIH.0000014641.68476.2d PMID: 14762323
7. Arnold M, Razum O and Coebergh JW (2010) Cancer risk diversity in non-western migrants to Europe: an overview of the literature Eur J Cancer 46(14) 2647–2659 DOI: 10.1016/j.ejca.2010.07.050 PMID: 20843493
8. Hemminki K and Li X (2002) Cancer risks in second-generation immigrants to Sweden Int J Cancer 99(2) 229–237 DOI: 10.1002/ijc.10323 PMID: 11979438
9. Lee J, Demissie K and Lu SE et al (2007) Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea Cancer Control 14(1) 78–85 PMID: 17242674
10. Li Y, Ahmad A and Kong D et al (2014) Recent progress on nutraceutical research in prostate cancer Cancer Metastasis Rev 33(2–3) 629–640 DOI: 10.1007/s10555-013-9478-9 PMID: 24375392 PMCID: 4074449
11. Pendleton JM, Tan WW and Anai S et al (2008) Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy BMC Cancer 8 132–2407-8–132 DOI: 10.1186/1471-2407-8-132 PMID: 18471323 PMCID: 2394534
12. King A, Young G (1999) Characteristics and occurrence of phenolic phytochemicals J Am Diet Assoc 99(2) 213–218 DOI: 10.1016/S0002-8223(99)00051-6 PMID: 9972191
13. Cheng T, Lu J and Wang J et al Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC-3 by flavonoids and saponins prepared from gynostemma pentaphyllum J Agric Food Chem 59(20) 11319–11329 PMID: 21905716
14. Knowles LM, Zigrossi DA and Tauber RA et al (2000) Flavonoids Suppress Androgen-Independent Human Prostate Tumor Proliferation Nutr Cancer 38(1) 116–122 PMID: 11341036
15. Aalinkeel R, Bindukumar B and Reynolds JL et al (2008) The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90 Prostate 68(16) 1773–1789 DOI: 10.1002/pros.20845 PMID: 18726985 PMCID: 2826114
16. Lee D, Szczepanski M and Lee YJ (2008) Role of bax in quercetin-induced apoptosis in human prostate cancer cells Biochem Pharmacol 75(12) 2345–2355 DOI: 10.1016/j.bcp.2008.03.013 PMID: 18455702 PMCID: 3266687
17. Szliszka E, Helewski KJ and Mizgala E et al (2011) The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells Int J Oncol 39(4) 771–779 PMID: 21743964
18. Chien C, Shen K and Huang J et al (2010) Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells Mol Cell Biochem 333(1) 169–180 DOI: 10.1007/s11010-009-0217-z
19. Senthilkumar K, Elumalai P and Arunkumar R et al (2010) Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3) Mol Cell Biochem 344(1) 173–184 DOI: 10.1007/s11010-010-0540-4 PMID: 20658310
20. Senthilkumar K, Arunkumar R and Elumalai P et al (2011) Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3) Cell Biochem Funct 29(2) 87–95 DOI: 10.1002/cbf.1725 PMID: 21308698
21. Bracarda S, de Cobelli O and Greco C et al (2005) Cancer of the prostate Crit Rev Oncol Hematol 56(3) 379–396 DOI: 10.1016/j.critrevonc.2005.03.010 PMID: 16310371
22. Taplin ME and Balk SP (2004) Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence J Cell Biochem 91(3) 483–490 DOI: 10.1002/jcb.10653 PMID: 14755679
23. Bennett NC, Gardiner RA and Hooper JD et al (2010) Molecular cell biology of androgen receptor signalling Int J Biochem Cell Biol 42(6) 813–827 DOI: 10.1016/j.biocel.2009.11.013
24. Koochekpour S (2010) Androgen receptor signaling and mutations in prostate cancer Asian J Androl 12(5) 639–657 DOI: 10.1038/aja.2010.89 PMID: 20711217 PMCID: 3006239
25. Thompson IM, Goodman PJ and Tangen CM et al (2003) The influence of finasteride on the development of prostate cancer N Engl J Med 349(3) 215–224 PMID: 12824459
26. Redman MW, Tangen CM and Goodman PJ et al (2008) Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach Cancer Prev Res (Phila) 1(3) 174–181 DOI: 10.1158/1940-6207.CAPR-08-0092
27. Andriole GL, Bostwick DG and Brawley OW et al (2010) Effect of dutasteride on the risk of prostate cancer N Engl J Med 362(13) 1192–1202 PMID: 20357281
28. Hiipakka RA, Zhang HZ and Dai W et al (2002) Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols Biochem Pharmacol 63(6) 1165–1176 DOI: 10.1016/S0006-2952(02)00848-1 PMID: 11931850
29. Gasmi J and Sanderson JT (2010) Growth inhibitory, antiandrogenic, and pro-apoptotic effects of punicic acid in LNCaP human prostate cancer cells J Agric Food Chem DOI: 10.1021/jf103306k PMID: 21067181
30. Kampa M, Hatzoglou A and Notas G et al (2000) Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines Nutr Cancer 37(2) 223–233 DOI: 10.1207/S15327914NC372_16
31. Ma Z, Nguyen TH and Huynh TH et al (2004) Reduction of rat prostate weight by combined quercetin-finasteride treatment is associated with cell cycle deregulation J Endocrinol 181(3) 493–507 DOI: 10.1677/joe.0.1810493 PMID: 15171697
32. Khan N, Asim M and Afaq F et al (2008) A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice Cancer Res 68(20) 8555–8563 DOI: 10.1158/0008-5472.CAN-08-0240 PMID: 18922931 PMCID: 2954499
33. Morris JD, Pramanik R and Zhang X et al (2006) Selenium- or quercetin-induced retardation of DNA synthesis in primary prostate cells occurs in the presence of a concomitant reduction in androgen-receptor activity Cancer Lett 239(1) 111–122 DOI: 10.1016/j.canlet.2005.07.037
34. Han HY, Shan S and Zhang X et al (2007) Down-regulation of prostate specific antigen in LNCaP cells by flavonoids from the pollen of Brassica napus L. phytomedicine 14(5) 338–343
35. Hsieh TC and Wu JM (2009) Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin Anticancer Res 29(10) 4025–4032 PMID: 19846946 PMCID: 3641843
36. Xing N, Chen Y and Mitchell SH et al (2001) Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells Carcinogenesis 22(3) 409–414 DOI: 10.1093/carcin/22.3.409 PMID: 11238180
37. Maggiolini M, Vivacqua A and Carpino A et al (2002) The mutant androgen receptor T877A mediates the proliferative but not the cytotoxic dose-dependent effects of genistein and quercetin on human LNCaP prostate cancer cells Mol Pharmacol 62(5) 1027–1035 DOI: 10.1124/mol.62.5.1027 PMID: 12391264
38. Yuan H, Gong A and Young CY (2005) Involvement of transcription factor Sp1 in quercetin-mediated inhibitory effect on the androgen receptor in human prostate cancer cells Carcinogenesis 26(4) 793–801 DOI: 10.1093/carcin/bgi021 PMID: 15661808
39. Yuan H, Pan Y and Young CY (2004) Overexpression of c-Jun induced by quercetin and resverol inhibits the expression and function of the androgen receptor in human prostate cancer cells Cancer Lett 213(2) 155–163 DOI: 10.1016/j.canlet.2004.04.003 PMID: 15327830
40. Yuan H, Young C and Tian Y et al (2010) Suppression of the androgen receptor function by quercetin through protein–protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells Mol Cell Biochem 339(1) 253–262 DOI: 10.1007/s11010-010-0388-7 PMID: 20148354
41. Saporita AJ, Ai J and Wang Z (2007) The Hsp90 inhibitor, 17-AAG, prevents the ligand-independent nuclear localization of androgen receptor in refractory prostate cancer cells Prostate 67(5) 509–520 DOI: 10.1002/pros.20541 PMID: 17221841 PMCID: 2810394
42. Ghosh PM, Malik S and Bedolla R et al (2003) Akt in prostate cancer: possible role in androgen-independence Curr Drug Metab 4(6) 487–496 DOI: 10.2174/1389200033489226 PMID: 14683476
43. Wang S, DeGroff VL and Clinton SK (2003) Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase J Nutr 133(7) 2367–2376 PMID: 12840208
44. Suh Y, Afaq F and Khan N et al (2010) Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells Carcinogenesis 31(8) 1424–1433 DOI: 10.1093/carcin/bgq115 PMID: 20530556 PMCID: 2915634
45. Kumar R, Verma V and Jain A et al (2011) Synergistic chemoprotective mechanisms of dietary phytoestrogens in a select combination against prostate cancer J Nutr Biochem 22(8) 723–731 DOI: 10.1016/j.jnutbio.2010.06.003
46. Baiz D, Pinder TA and Hassan S et al (2012) Synthesis and characterization of a novel prostate cancer-targeted phosphatidylinositol-3-kinase inhibitor prodrug J Med Chem 55(18) 8038–8046 DOI: 10.1021/jm300881a PMID: 22924393 PMCID: 3738169
47. Sak K (2014) Cytotoxicity of dietary flavonoids on different human cancer types Pharmacogn Rev 8(16) 122–146 DOI: 10.4103/0973-7847.134247 PMID: 25125885 PMCID: 4127821
48. Sak K (2014) Site-specific anticancer effects of dietary flavonod quercetin Nutr Cancer 66(2) 177–193 DOI: 10.1080/01635581.2014.864418 PMID: 24377461
49. Tang SN, Singh C and Nall D et al (2010) The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition J Mol Signal 5 14–2187-5–14 DOI: 10.1186/1750-2187-5-14 PMID: 20718984 PMCID: 2933702
50. Ma ZS, Huynh TH and Ng CP et al (2013) Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro Food Chem 138(1) 48–53 DOI: 10.1016/j.foodchem.2012.09.102
51. Ma ZS, Huynh TH and Ng CP et al (2004) Reduction of CWR22 prostate tumor xenograft growth by combined tamoxifenquercetin treatment is associated with inhibition of angiogenesis and cellular proliferation Int J Oncol 24(5) 1297–1304 PMID: 15067354






