Management of dermatologic toxicities related to epidermal growth factor receptor inhibitor therapy across Europe: can we get a consensus?
L Lemmens1
1Department of Gastroenterology, University Hospitals Leuven, Leuven, Belgium
Correspondence to: L Lemmens. Email: liesbeth.lemmens@uz.kuleuven.ac.be
Abstract
To date, there are no phase III trial data that can guide healthcare professionals in managing toxicities of epidermal growth factor receptor inhibitors (EGFRIs). This exploratory survey assessed the similarities and differences in nursing management of EGFRI-related toxicities across 10 European countries. A questionnaire that was sent to ten nurses who specialize in the treatment of patients with EGFRI asked about the prevention and treatment of acneiform rash, dry skin/eczema, fissures, paronychia, and pyogenic granuloma. Responses from seven nurses showed that overall (with the exception of rash), treatment differed markedly between countries in the management of dermatologic toxicities. These substantial differences across the European hospitals surveyed suggest that it might be worthwhile to develop treatment algorithms by conducting a consensus conference or a follow-up survey with several assessments and a larger sample.
Cancer treatment is being transformed with the advent of entirely new classes of drugs that specifically inhibit genes, proteins, or processes involved in cancer development, thus sparing a greater number of healthy cells. One cancer drug target is the epidermal growth factor receptor (EGFR, also called ErbB1 and human epidermal growth factor receptor 1 (HER 1)). EGFR is a member of a protein family called receptor tyrosine kinases, which control vital cell processes such as proliferation, migration, and survival [1]. EGFR has been linked to many common cancers, including head and neck cancer, colorectal cancer (CRC), pancreatic cancer, and non-small-cell lung cancer (NSCLC), and overproduction of EGFR is associated with more aggressive clinical behavior (including more tumor angiogenesis, proliferation, and metastasis) and shorter overall survival time [2–4]. Based on their efficacy in clinical trials, therapies that inhibit the EGFR signaling pathway, such as the small-molecule inhibitors, erlotinib (Tarceva®) and gefitinib (Iressa®), and the monoclonal antibodies, cetuximab (Erbitux®) and panitumumab (Vectibix®), are now routinely used in clinical practice to control these cancers [5–8].
Several types of epithelial cells, such as those in the epidermis, hair follicles, sebaceous glands, mucosal tissues, and ocular tissues, produce high levels of EGFR. Epidermal growth factor receptor inhibitors (EGFRIs) are thus commonly associated with class-specific toxicities, many of which are new to oncology teams and differ from the toxicities regularly observed with conventional chemotherapy regimens [9]. Skin toxicities, such as acneiform rash, dry skin, fissures, and nail and hair disorders, are commonly associated with treatment with EGFRIs [10,11]. To date, there are no data from phase III trials that can guide healthcare professionals in managing toxicities of EGFRIs.
In this article, a first step towards improving and standardizing patient care is reported. In brief, an exploratory survey of nurses from different European countries was conducted to assess the similarities and differences in the management of EGFRI-related toxicities across European countries. Between April and November 2009, a questionnaire was sent to ten hospital-based clinical nurse specialists who focus on the treatment of patients with EGFRI; these nurses were selected based on their prior participation in a nursing advisory board on skin toxicities of EGFRIs or membership in the European Oncology Nursing Society TARGET task force on targeted therapies. The questionnaire asked about the prevention and treatment of EGFRI-related dermatologic toxicities including acneiform rash, dry skin/eczema, fissures, nail changes such as paronychia, and pyogenic granuloma. All questions were open-ended to allow for the possibility of discovering management approaches that are not reported in the literature. Seven nurses responded, from Belgium, Ireland, the Netherlands, Spain, Sweden, Switzerland and the United Kingdom. As shown in the tables and discussed in detail below, approaches to management of EGFRI toxicities differed substantially across the nurses surveyed.
Skin rash
All survey respondents provided patient education and general skin care advice, including the use of moisturizers (Table 1), but they also reported that they do not provide routine pharmaceutical prophylaxis for skin rash. In some countries, topical antibiotic creams are given immediately with the appearance of grade 1 acneiform rash, while in others oral antibiotics are preferred. Treatment of grade 3 acneiform rash varies greatly, with oral/topical steroids as well as antibiotics and antihistamines being incorporated into some strategies. Overall, there appeared to be substantial variation in the management of skin toxicities across Europe. These results are consistent with another survey that inquired about the management of EGFRI-associated skin rash across 110 oncology practices in the United States, which also detected pronounced diversity in the types of interventions used [12].
Table 1:
Prevention and treatment of acneiform rash
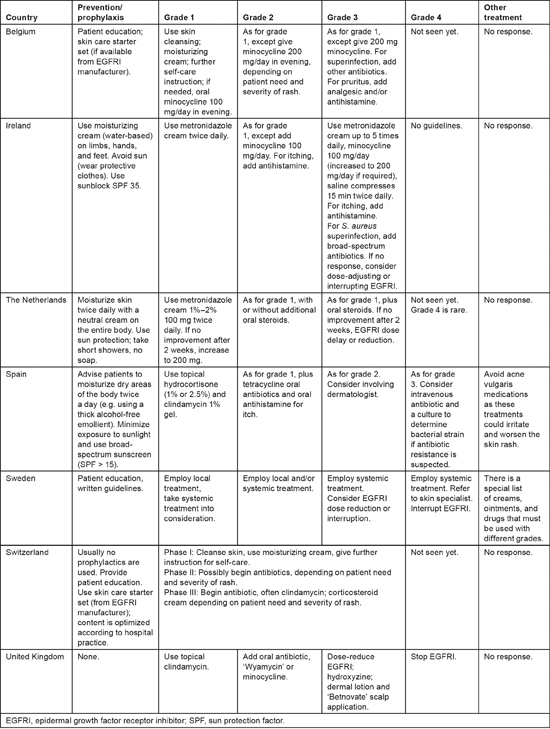
An interdisciplinary, international panel has recommended dividing papulopustular reactions into three categories, based on the severity of symptoms, the impact of symptoms on daily activities, and whether the patient has signs of, or potential for, superinfection [13,14]. According to the international guidelines, if there is no response to rash treatment within 2—4 weeks, EGFRI therapy should be temporarily suspended as directed by the manufacturer [13,14]. Therapy can be restarted once the rash adequately diminishes in severity. Referral to a dermatologist is warranted if lesions have an uncharacteristic appearance or distribution; if there is necrosis, blistering, or petechial/purpuric lesions; or if patients have atypical dermatological manifestations unrelated to rash [15]. However, consistent with the findings of this survey, all recommendations are based on experience rather than clinical trial data, and there are important geographic variations in actual management of EGFRI skin toxicity [11].
Dry skin
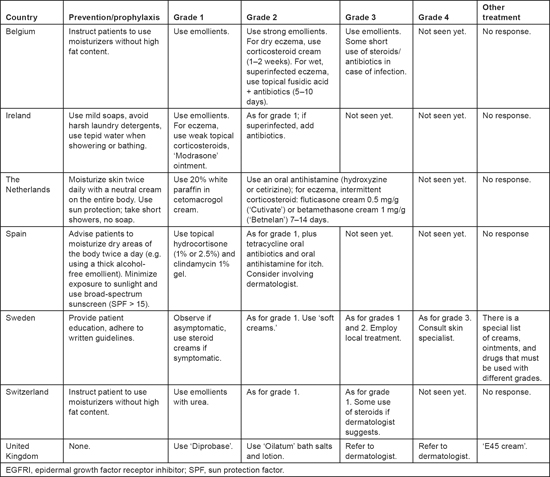
Various approaches to preventing dry skin were recommended, but the use of emollients and protection against the sun were among the most common (Table 2). Management approaches differed from each other with regard to the types of emollients to be used; whether and when to use corticosteroids; and the need for, and timing of, referral to a dermatologist. Not all respondents mentioned examining patients for the possibility of infection, although it could be assumed that these evaluations are conducted when infections are suspected.
Table 2:
Prevention and treatment of dry skin/eczema
Fissures
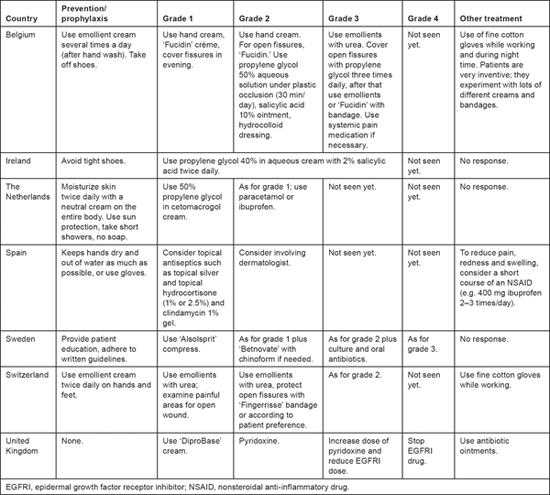
For fissures, there was virtually no overlap in the therapeutic measures reported, although two respondents reported using propylene glycol for grade 1 fissures. Four respondents reported giving pain medication, but the timing of its initiation varied. Only two respondents reported experience with grade 4 fissures. In one of these centers reduced dose or interrupted EGFRI therapy for severe fissures. Common recommendations for the treatment of fissures in Europe include a 50% solution of propylene glycol in water, an ointment containing 10% salicylic acid, or a hydrocolloid dressing or liquid cyanoacrylate glue [11]. Patient education also differed widely among the respondents, according to whether prophylactic care is recommended for the entire body, just the hands and feet, just the hands, or just the feet (see Table 3).
Table 3:
Prevention and treatment of fissures
Paronychia
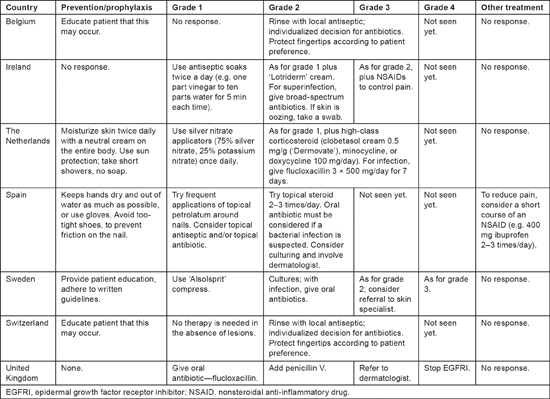
Treatment of paronychia among the respondents varied even more markedly than treatment of the other dermatologic toxicities (see Table 4). For grade 1 paronychia, management strategies ranged from observation to the use of an oral antibiotic. Most respondents gave some sort of topical treatment for grade 1; two added a corticosteroid for grade 3. Antibiotic therapy was given if needed. Three respondents considered referral to a dermatologist for moderate or severe paronychia. Only two respondents reported experience with grade 4 paronychia; EGFRI therapy was routinely stopped for grade 4 paronychia at one of the centers.
Table 4:
Prevention and treatment of paronychia
Pyogenic granuloma
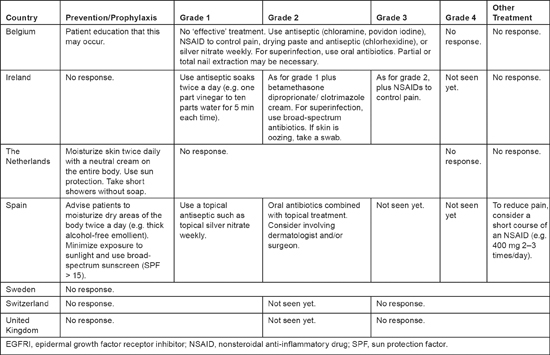
Only three respondents reported experience with any grade of pyogenic granulomas because this condition is rarely observed (see Table 5). One nurse felt that no effective treatment was available for this condition. For grade 1 pyogenic granulomas, the three respondents recommended various antiseptic soaks; they recommended adding topical treatment and oral antibiotics (if superinfections were present) for grade 2. A short course of nonsteroidal anti-inflammatory drugs was also suggested to alleviate pain. For prophylactic care, three nurses advised patients to moisturize their bodies twice daily with an alcohol-free emollient and to minimize exposure to the sun or use broad-spectrum sun protection. One nurse also recommended that patients take short showers without soap.
Table 5:
Prevention and treatment of pyogenic granuloma
Conclusion
This pilot survey of European hospitals revealed that there is no consensus on the management of EGFRI-related skin toxicities, but respondents clearly agreed that these toxicities need treatment. This is not surprising, given that treatment options described in the medical literature are based on case reports, case series, or reported personal experience, not on data from large, randomized, controlled trials. Other potential explanations for differences in management are that (a) the specific approved EGFRIs and their indications vary between countries in Europe and (b) different agents have slightly different toxicity profiles.
Despite the diversity in their answers, the respondents clearly recognized that EGFRI toxicities are treatable and require medical intervention. While dermatologic reactions are usually mild to moderate in severity, they are sometimes physically or emotionally bothersome such that patients wish to discontinue therapy. Conversely, patients may under-report their symptoms out of fear that the anticancer therapy will be withheld. Therefore, it is important that healthcare professionals minimize the incidence and severity of side effects, prevent their progression, and thus the need for dose reduction or discontinuation of potentially effective treatment.
Standardized consensus guidelines to prevent or treat EGFRI-related dermatologic toxicities may help improve patient care and be a useful resource for clinicians throughout Europe. These guidelines should include detailed information on how to educate patients about lifestyle modifications, early signs of toxicity, and the importance of treatment adherence; how to regularly assess patients' side effects and related quality of life; and how to provide resources for psychosocial support. Assessment or patient self-report of side effects should begin within 14 days from EGFRI initiation [7].
This survey was exploratory, and the results should be interpreted in the context of certain limitations. The survey is not representative of the entire European Union or of all hospitals within each country. A more detailed follow-up survey with an expanded questionnaire is planned in order to facilitate the development of a consensus statement and, potentially, algorithms for the optimal treatment of toxicities. In the meantime, management of EGFRI-related toxicities should be undertaken on a case-by-case basis, with an emphasis on preventing dose modification whenever possible. Effective management and patient education may help to alleviate the significant social and emotional anxiety related to these manageable toxicities, thus resulting in improved quality of life.
Disclosures
The author has served as a consultant for and has received honoraria and travel grants from Amgen (Europe) GmbH.
Acknowledgments
The author would like to thank the following nurses for their participation in the survey: Ada Kinneally, Clinical Nurse Manager, Oncology Department, Waterford Regional Hospital, Ireland; Anita Margulies, Clinical Nurse and Lecturer, Oncology Clinic, University Hospital Zurich, Switzerland; Clementine Molin, Head of Clinical Trial Unit, Oncology Clinic, Karolinska University Hospital, Stockholm, Sweden; Christine Boers-Doets, Waterlandziekenhuis, Trial Office Oncology, Purmerend, the Netherlands; Elena Elez Fernandez, Gastrointestinal Cancer Program, Medical Oncology Service, Vall d’Hebron University Hospital, Barcelona, Spain; Maggie Uzzell, Royal Marsden Hospital, London, United Kingdom. Medical writing and editorial support (funded by Amgen (Europe) GmbH) was provided by Supriya Srinivasan, PhD, of Scientia Medical Communications, LLC.
References
1. Yarden Y (2001) The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 37
2. Yarden Y,
3. Galizia G,
4. Spano JP,
5.
6.
7.
8.
9. Kang SP,
10. Melosky B,
11. Segaert S,
12. Boone SL,
13. Eaby B,
14. Lynch TJ
15. Pérez-Soler R,