eHealth tools to improve the health-related quality of life of family caregivers of cancer patients in developing countries: a systematic review protocol
Israel Gabriel1,a , Andy Emmanuel2,b , P Pratitha1, Divitha Subramanian1, Thilaka Chandanee1 and Fariba Hosseinisazi1
1Institute of Health and Management, Sydney 2150, Australia
2Community Education Instructor, Queensland Ambulance Service, Brisbane, QLD 4207, Australia
ahttps://orcid.org/0000-0002-5663-450X
bhttps://orcid.org/0000-0002-8563-4306
Abstract
Background: ehealth improves the health-related quality of life for cancer patients and their families by providing easier access to medical information, promoting self-management, and providing personalised care through digital platforms. However, there is still a dearth of comprehensive understanding of their influences in developing countries.
Objectives: To identify several ehealth interventions accessible to family caregivers of people with cancer in developing countries and to assess the impact of these interventions on their health-related quality of life.
Methods: The review will adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols. Health databases and search engines, including PubMed, Medline via Ovid, Embase via Ovid, and CINAHL via EBSCOhost, will be used. We will include quantitative or mixed method research evaluating e-health, with a particular emphasis on the health-related quality of life of family caregivers of cancer patients. Studies from developing countries, including peer-reviewed journals and grey literature, will be considered without regard to publication date.
The study selection process involves screening titles and abstracts for relevance, then, doing a full-text assessment against the inclusion criteria. The Cochrane Risk of Bias tool for randomised controlled trials and ROBINS-I V2 for non-randomised studies will be employed to assess the quality of the included studies. The A Measurement Tool to Assess Systematic Reviews will assess the quality of this systematic review.
Implications: This review offers a thorough and impartial summary of current research on e-health tools and their impact on the health-related quality of life of family caregivers of cancer patients. It seeks to inform evidence-based decision-making across healthcare, policy development, and research design by identifying knowledge gaps, emphasising areas requiring further investigation, and steering future research.
Systematic review registration: PROSPERO CRD42024622302
https://www.crd.york.ac.uk/PROSPERO/view/CRD42024622302
Keywords: e-health, telehealth, telemedicine, quality of life, well-being, family caregivers of cancer patient, informal caregivers, developing countries
Correspondence to: Israel Gabriel
Email: israel@ihm.edu.au
Published: 17/10/2025
Received: 03/03/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Cancer constitutes a significant global health burden [1]. In 2022, the worldwide cancer burden was at 20 million individuals, resulting in 9.7 million cancer-related deaths [2]. By 2050, almost 35 million new cancer cases are anticipated, reflecting a significant 77% rise from the estimated 20 million cases recorded in 2022 [3]. The population's growth and the number of people reaching advanced ages, along with shifts in the distribution and prevalence of the key risk factors for cancer, many of which are linked to socioeconomic development, are the main causes of the increase in new cancer cases [4]. Notwithstanding these challenges, the population of cancer survivors is steadily increasing, with improvements in cancer survival rates attributable to advancements in technology and pharmacological research in cancer treatment [5, 6]. A considerable number of individuals who have survived cancer are required to navigate the physical effects of the disease and its treatment, which may result in functional and cognitive challenges, alongside a range of psychological, social, spiritual and economic consequences [7–9].
During cancer treatment, patients become more reliant on others, have diminished performance and face heightened physical and psychological challenges [10]. Consequently, family caregivers are an integral part of the caregiving continuum, significantly contributing to the support of these patients [11]. Family caregivers often provide extended, intensive personal care without respite, compensation or external support. Providing care and support during this period can be a difficult task [12]. Many family caregivers prioritise the needs and emotions of their loved ones with cancer over their own [13]. Physical care, assistance with daily tasks, medication administration, transportation, emotional support, household chores and companionship are just a few of the responsibilities they do [14]. Likewise, family caregivers engage in care and treatment decisions while monitoring treatment side effects and symptoms with diligence [15]. Nonetheless, providing comprehensive care and support to family members may adversely affect the psychological, social, physical and spiritual well-being and overall health-related quality of life (HRQoL) of family caregivers, potentially endangering their health [16, 17]. HRQoL is a subjective assessment that encapsulates an individual's perceptions and emotions regarding their health and its impact on daily living. It is a multifaceted concept that evaluates the impact of health, disease or injury on an individual's overall well-being and life satisfaction. It pertains not just to the lack of sickness but also to physical, mental and social well-being [18].
There has been a lot of interest in developing focused interventions to meet the needs of cancer patients' family caregivers [16, 19–21]. A substantial amount of research has recorded the effect of interventions on the HRQoL of family caregivers of people living with cancer [20–23]. For example, Chow et al [22] conducted a systematic review and meta-analysis to investigate the effectiveness of interventions designed to support caregivers of patients with advanced cancer, focusing on caregivers' QoL and mental health outcomes. The review encompassed 56 articles that reported on randomised controlled trials (RCTs), mostly involving psychoeducational or problem-solving interventions. The interventions demonstrated significant effects on overall QoL, mental well-being, anxiety and depression in comparison to standard care. Ahn et al [20] similarly reported in a systematic review encompassing 11 studies, with interventions categorised as psychological, educational or a combination of both. Most interventions had statistically significant outcomes in reducing psychological distress and caregiving burden, while improving QoL, self-efficacy and caregiving competence. Conversely, Kalyani et al [23], in their systematic review and meta-analysis involving eight studies to examine the effects of psychosocial interventions on the QoL of cancer caregivers, reported that while these interventions were found to improve caregivers' QoL, but not statistically significantly.
eHealth is another significant resource for meeting the care needs of cancer patients' family caregivers, particularly in developed countries with well-established and stable healthcare systems facilitated by eHealth [16, 24, 25]. eHealth in oncology includes psychoeducational programs, remote symptom monitoring, self-care education, online peer support forums, physical activity tools and various other applications using digital platforms [14, 26]. eHealth may significantly improve healthcare delivery through prevention, early diagnosis, medication safety, treatment adherence by patients, guideline adherence by providers, medication safety, improved care coordination, documentation, data management and research, among others [27–29]. eHealth has the potential to enhance cancer care delivery and research, hence reducing cancer-related morbidity and mortality in developing countries, if it is embraced and effectively implemented throughout the cancer continuum [30]. However, this is not true in many developing countries, where eHealth has considerable gaps in acceptance and effectiveness [31]. These gaps include a lack of infrastructure, digital knowledge and resources, which impedes broad acceptance and equal access to eHealth technologies, as well as death of shortage of trained professionals, impede the integration of eHealth technology [32]. Moreover, there is a lack of published literature on the application of eHealth solutions in oncology in developing countries, particularly in African countries such as Nigeria, Ghana, Kenya and other sub-Saharan regions [33–36]. Most of the existing literature focuses on infectious diseases, including
HIV/AIDS, Malaria and Tuberculosis [37, 38]. Consequently, addressing these gaps is crucial for realising the potential of eHealth to improve cancer outcomes in developing countries. Thus, the purpose of this review was to identify eHealth interventions aimed at family caregivers of people living with cancer in developing countries and the effects on their HRQoL.
Review question(s)
- What eHealth interventions are accessible for family caregivers of cancer patients in developing countries?
- What is the effectiveness of these eHealth interventions in improving the HRQoL for this population?
Methods
Protocol and registration
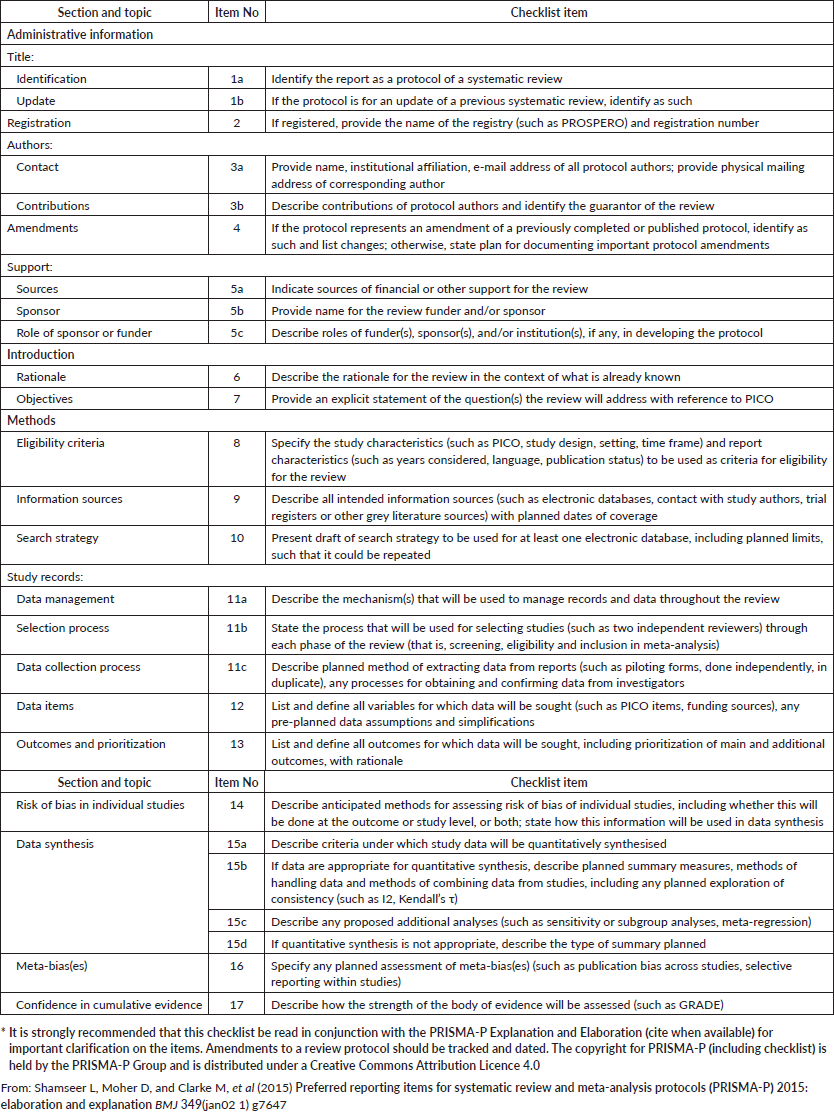
The systematic review will follow the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) statement to ensure that the protocol is complete and meets high standards [39, 40] (Supplementary file 1). The methodological approach encompasses three key components: the search strategy, the selection of relevant articles and the critical evaluation of their methodological quality. The review protocol is prospectively registered with the International Prospective Register of Ongoing Systematic Reviews (PROSPERO) database (registration number CRD42024622302). https://www.crd.york.ac.uk/PROSPERO/view/CRD42024622302
Eligibility criteria
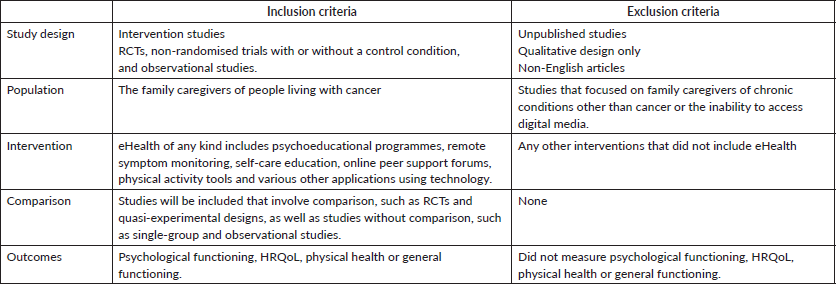
The eligibility criteria for this review will be determined using the Population, Intervention, Comparison, Outcome (PICO). framework. We will include studies that (a) are RCTs or non-randomised trials with or without a control condition or observational studies; (b) target unpaid family caregivers of cancer patients aged 18 years and older; (c) comprise eHealth interventions, such as psychoeducational programmes, remote symptom monitoring, self-care education, online peer support forums, physical activity tools and other technology-based applications; (d) report quantitative outcomes of family caregivers of cancer patients, such as psychological functioning, HRQoL, physical health and general functioning and (e) studies undertaken in English and other languages, published in countries designated by the United Nations as developing, characterised by low living standards, inadequate infrastructure and a predominantly non-industrialised economy [35] (Table 1).
Information sources
Health databases, specifically PubMed, Cochrane, Medline via Ovid, Embase via Ovid and CINAHL via EBSCOhost, will be meticulously searched for original articles. The search will cover scientific articles published in English and other languages from the inception of the databases until 2024. We will conduct manual searches of abstracts from the reference lists of pertinent articles to identify supplementary studies.
Search strategy
The search strategy will seek to identify published studies. This review will employ a three-phase search strategy. First, the preliminary search of PubMed was conducted to establish a fundamental understanding of the research landscape on the subject. Additionally, facilitate the refining of review questions and identify essential terminology. The terminology found in the titles and abstracts of pertinent articles will be used to characterise the articles and along with controlled vocabulary, a full search strategy will be developed for the full review. The search strategy, encompassing all selected keywords and index terms, will be tailored for each health database and/or information source. A librarian contributed to the development of the initial search strategies and will participate in the final search strategy and the management of search results.
Table 1. Inclusion and exclusion criteria.
The second phase will involve a simultaneous search to enhance the sensitivity of the results, following the completion of all identified keywords and index terms. The four health databases that will be searched are PubMed, Medline via Ovid, Embase via Ovid and CINAHL via EBSCOhost. Additionally, the associated platforms will be searched.
The third phase of the search strategy involves the screening of the reference lists of all included sources, which will be examined for supplementary studies. This review will exclusively encompass quantitative and mixed-method studies. Studies published in languages other than English will not be included; however, all publication years will be included.
Study records
Data management
We will use Covidence software to facilitate screening, data extraction, dispute resolution, author review and validation of data, development of data tables and creation of data files for future study use. Covidence is a leading tool for coordinating systematic reviews that is effectively utilised in various projects across several research fields, particularly healthcare [41].
Selection process
Records will be compiled and duplicates will be removed using EndNote 20 software. The data will be managed with Covidence software. The screening and selection procedure will adopt a three-phase approach to ensure precision and comprehensiveness.
A team of three reviewers will evaluate titles and abstracts using predefined inclusion and exclusion criteria. Two team members will independently evaluate titles and abstracts once all raters reach a 90% consensus.
The second phase is a comprehensive review of the full text of selected articles. Two independent reviewers will check the full text against the inclusion criteria and write down reasons for any articles they exclude, which will be carefully noted in the PRISMA-P flow diagram [42], as shown in Figure 1.
The third phase involves two independent reviewers deciding on the final inclusion of studies. We will address disagreements among reviewers either through discussion or by including additional team members. The results of the search and the studies that were included will be clearly explained in the systematic review and shown in a PRISMA 2020 flow diagram [40].
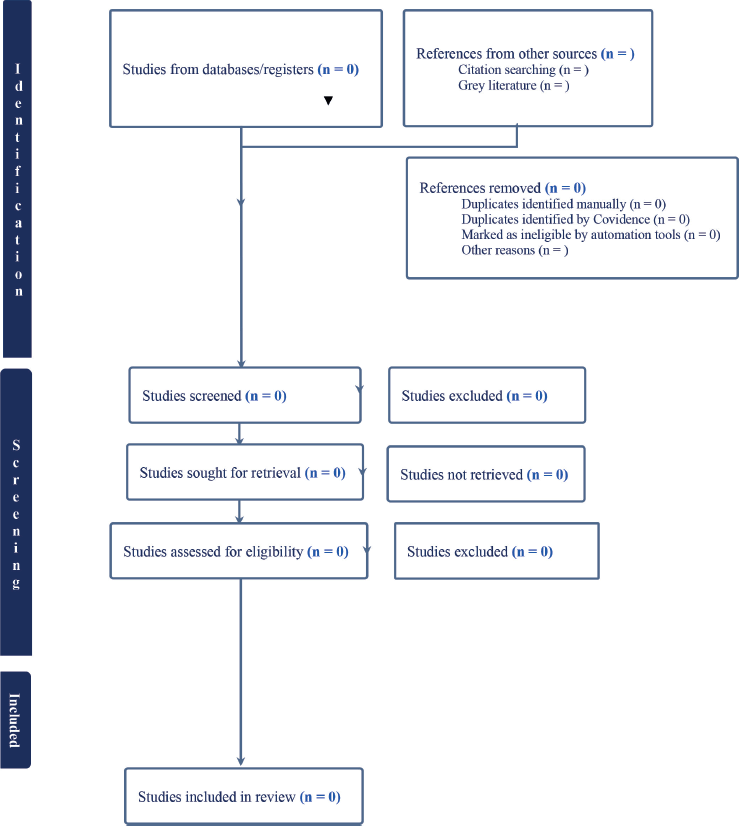
Figure 1. PRISMA flow diagram of study retrieval and inclusion process, adapted from (PRISMA, 2021).
Data collection
Two independent reviewers will methodically retrieve data from the included studies using Covidence software. We intend to extract data regarding publication information, conceptualisation components, methodologies and outcomes. We will extract author names, year of publication, journal and, if applicable, funding source from publication information. We will extract information on the study questions or hypotheses, theoretical or conceptual frameworks and types of interventions within the components of conceptualisation. Regarding the methodology, we will extract the study design, study setting, sampling and recruitment processes and data collection methods.
For the outcomes, we will extract research findings on interventions and their impact on HRQoL. To facilitate a methodical and coordinated approach to data extraction, at least two authors will extract from each article to ensure consistency. The two authors shall meet to deliberate and assess the extracted data for any missing information or conflicting reports. Independent authors shall convene with extractors to attain a consensus regarding any discrepancies in information, should the primary reviewers be unable to reach a definitive conclusion.
Risk of bias in individual studies
Three authors will independently use the Cochrane Risk of Bias tool for RCTs [43] and ROBINS-I V2 for non-randomised studies [44]. The Cochrane Risk of Bias tool evaluates the randomisation process, potential biases, allocation concealment, outcome assessment, reporting of results and attrition rates. ROBINS-I V2 evaluates possible bias across multiple domains, including exposure/intervention assignment, confounding variables, selection bias, information bias and attrition bias.
Assessment of methodological quality
Four reviewers will independently evaluate the quality of systematic reviews using the A Measurement Tool to Assess Systematic Reviews (AMSTAR) tool [45]. AMSTAR is an 11-item tool widely regarded as one of the most effective tools for assessing the methodological quality of systematic reviews, demonstrating strong face and content validity. The instrument allows researchers to assess the quality of a systematic review by examining aspects such as the literature search strategy, study selection process and analysis methods, thereby helping to determine the reliability of its findings [46].
Data synthesis
The evidence will be described using a narrative approach. The methodological rigour of the included studies will be evaluated to ascertain the potential effect of biases on the results, utilising a standard evaluation technique, followed by a synthesis of their findings. Furthermore, all authors will review all included articles independently before integrating the reviews and arriving at a consensus where there is conflicting or opposing outcome. This review focusses on the following domains: author, year, setting, theoretical framework, kind of eHealth, purpose, research design, response rate, data collection, outcome measures and findings. The included studies' methodological rigour will be evaluated using a standard assessment tool to establish the extent to which biases may influence. The domains of interest in this review includes author, year, setting, theoretical framework, type of eHealth, purpose, study design, response rate data collection, outcome measures and findings.
Care will be taken to ensure that any overlap between the primary studies included in the systematic reviews is accounted for and considered when deriving conclusions from the evidence. The limitations of the data and gaps in the evidence will also be highlighted.
Discussion
This systematic review will provide a thorough and effective review of the existing evidence regarding technology-based interventions aimed at enhancing HRQoL. The strengths of this systematic review will be found in its organised approach and extensive, comprehensive search strategy, quality assessment and data analysis. This approach will identify all relevant literature to assess the effectiveness of eHealth tools in enhancing the HRQoL for family caregivers of cancer patients in developing countries. This will guide future intervention policies and commissioning decisions. Furthermore, it will identify areas of weakness, inconsistency and gaps in the evidence base for eHealth tools aimed at enhancing HRQoL concerning secondary evidence, thereby guiding future research efforts. The work will emphasise both the areas where well-conducted systematic reviews have revealed weaknesses in the primary evidence, as well as the weaknesses of the secondary evidence, particularly regarding low-quality systematic reviews.
Ethics and dissemination
Since this is a review of original studies previously approved by research ethics committees, it is unnecessary to submit the review to the assessment of ethical aspects involving human beings. The policies and practices of healthcare institutions and governmental health organisations may be influenced by the results of this study, which will be disseminated through peer-reviewed publications, webinars and conferences. Additionally, we will employ the data to create new studies that resolve the identified academic gaps.
Conflicts of interest
No conflicts of interest.
Funding
There is no funding for this review protocol.
References
1. Hughes T, Harper A, and Gupta S, et al (2024) The current and future global burden of cancer among adolescents and young adults: a population-based study Lancet Oncol 25(12) 1614–1624 https://doi.org/10.1016/S1470-2045(24)00523-0 PMID: 39557059
2. Bray F, Laversanne M, and Sung H, et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA: Cancer J Clin 74(3) 229–263 PMID: 38572751
3. Bizuayehu HM, Ahmed KY, and Kibret GD, et al (2024) Global disparities of cancer and its projected burden in 2050 JAMA Network Open 7(11) e2443198-e https://doi.org/10.1001/jamanetworkopen.2024.43198
4. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA: Cancer J Clin 71(3) 209–249 PMID: 33538338
5. Gabriel I, Creedy D, and McGuire A, et al (2022) Improving the health-related quality of life of adult Nigerians living with cancer and their family caregivers: intervention development Pilot Feasibility Stud 8(1) 151 https://doi.org/10.1186/s40814-022-01117-w PMID: 35859142 PMCID: 9297656
6. Miller KD, Nogueira L, and Devasia T, et al (2022) Cancer treatment and survivorship statistics, 2022 CA: Cancer J Clin 72(5) 409–436 PMID: 35736631
7. Gabriel IO and Mayers PM (2019) Effects of a psychosocial intervention on the quality of life of primary caregivers of women with breast cancer Eur J Oncol Nurs 38 85–91 https://doi.org/10.1016/j.ejon.2018.12.003 PMID: 30717942
8. Jabeen S, Zakar R, and Zakar MZ, et al (2024) Experiences of family caregivers in dealing with cases of advanced breast cancer: a qualitative study of the sociocultural context in Punjab, Pakistan BMC Public Health 24(1) 1030 https://doi.org/10.1186/s12889-024-18404-1 PMID: 38609905 PMCID: 11015732
9. Gabriel I, Creedy D, and Coyne E (2021) Feasibility of a socio-spiritual intervention to improve quality of life of adult Nigerians with cancer and their family caregivers: Protocol for a randomised controlled trial Contemp Clin Trials Commun 22 100802 https://doi.org/10.1016/j.conctc.2021.100802 PMID: 34195469 PMCID: 8233201
10. Rezaei M, Keyvanloo Shahrestanaki S, and Mohammadzadeh R, et al (2024) Caregiving consequences in cancer family caregivers: a narrative review of qualitative studies Front Public Health 12 1334842 https://doi.org/10.3389/fpubh.2024.1334842 PMID: 38584929 PMCID: 10997218
11. Cho E, Yang Y, and Kim HK, et al Priorities of family caregivers in preserving functional abilities of individuals with alzheimer’s disease living at home: a best-worst scaling approach Res Community Public Health Nurs 35(2) 156–167
12. Becqué YN, Rietjens JA, and van der Heide A, et al (2021) How nurses support family caregivers in the complex context of end-of-life home care: a qualitative study BMC Palliat Care 20(1) 162 https://doi.org/10.1186/s12904-021-00854-8 PMID: 34657623 PMCID: 8521979
13. Breuning M, Mählmann S, and Kerek‐Bodden H, et al (2024) Family caregivers of cancer patients: burdens and support preferences of partner, parent and adult‐child caregivers Psycho‐Oncology 33(9) e9310 https://doi.org/10.1002/pon.9310
14. Darley A, Coughlan B, and Furlong E (2021) People with cancer and their family caregivers’ personal experience of using supportive eHealth technology: a narrative review Eur J Oncol Nurs 54 102030 https://doi.org/10.1016/j.ejon.2021.102030
15. Bechthold AC, Azuero A, and Puga F, et al (2023) What is most important to family caregivers when helping patients make treatment-related decisions: findings from a national survey Cancers 15(19) 4792 https://doi.org/10.3390/cancers15194792 PMID: 37835486 PMCID: 10572058
16. Shaffer KM, Turner KL, and Siwik C, et al (2023) Digital health and telehealth in cancer care: a scoping review of reviews Lancet Digital Health 5(5) e316-e327 https://doi.org/10.1016/S2589-7500(23)00049-3 PMID: 37100545 PMCID: 10124999
17. Uzun U, Başar S, and Saritaş A (2024) Spiritual needs of family caregivers in palliative care BMC Palliat Care 23(1) 256 https://doi.org/10.1186/s12904-024-01589-y PMID: 39511622 PMCID: 11542245
18. Yin S, Njai R, and Barker L, et al (2016) Summarizing health-related quality of life (HRQOL): development and testing of a one-factor model Popul Health Metrics 14(1) 22 https://doi.org/10.1186/s12963-016-0091-3
19. Ahmed S, Naqvi SF, and Sinnarajah A, et al (2020) Patient and caregiver experiences with advanced cancer care: a qualitative study informing the development of an early palliative care pathway BMJ Support Palliat Care 14(e1) e790-e797
20. Ahn S, Romo RD, and Campbell CL (2020) A systematic review of interventions for family caregivers who care for patients with advanced cancer at home Patient Educ Couns 103(8) 1518–1530 https://doi.org/10.1016/j.pec.2020.03.012 PMID: 32201172 PMCID: 7311285
21. Gabriel IO, Creedy DK, and McGuire A, et al (2025) Feasibility and preliminary effects of a socio-spiritual intervention for adults with cancer and their family caregivers: a pilot randomised controlled trial ecancermedicalscience 19 1851 https://doi.org/10.3332/ecancer.2025.1851 PMID: 40259903 PMCID: 12010129
22. Chow R, Mathews JJ, and Cheng EY, et al (2023) Interventions to improve outcomes for caregivers of patients with advanced cancer: a meta-analysis JNCI: J Natl Cancer Inst 115(8) 896–908 https://doi.org/10.1093/jnci/djad075 PMID: 37279594 PMCID: 10407714
23. Kalyani CV, Rohilla KK, and Gupta P, et al (2023) Effect of psychosocial interventions on cancer’s caregiver quality of life: meta-analysis Clin Pract Epidemiol Ment Health: CP & EMH 19 e174501792308240 https://doi.org/10.2174/17450179-v19-e230927-2022-HT14-4336-1
24. Heynsbergh N, Heckel L, and Botti M, et al (2018) Feasibility, useability and acceptability of technology-based interventions for informal cancer carers: a systematic review BMC Cancer 18(1) 244 https://doi.org/10.1186/s12885-018-4160-9 PMID: 29499663 PMCID: 5834845
25. Kent EE, Tan KR, and Nakamura ZM, et al (2024) Building on and tailoring to: adapting a cancer caregiver psychoeducational intervention for rural settings Cancer Med 13(17) e70187 https://doi.org/10.1002/cam4.70187 PMID: 39234997 PMCID: 11375528
26. Doyle-Lindrud S (2020) State of eHealth in cancer care: review of the benefits and limitations of eHealth tools Clin J Oncol Nurs 24 10–15. https://doi.org/10.1188/20.CJON.S1.10-15 PMID: 32441698
27. Eden R, Burton-Jones A, and Scott I, et al (2018) Effects of eHealth on hospital practice: synthesis of the current literature Aust Health Rev 42(5) 568–578 https://doi.org/10.1071/AH17255 PMID: 29986809
28. Elbert NJ, van Os-Medendorp H, and van Renselaar W, et al (2014) Effectiveness and cost-effectiveness of ehealth interventions in somatic diseases: a systematic review of systematic reviews and meta-analyses J Med Internet Res 16(4) e2790 https://doi.org/10.2196/jmir.2790
29. Marcolino MS, Oliveira JAQ, and D’Agostino M, et al (2018) The impact of mHealth interventions: systematic review of systematic reviews JMIR mHealth and uHealth 6(1) e8873 https://doi.org/10.2196/mhealth.8873
30. Campanella P, Lovato E, and Marone C, et al (2016) The impact of electronic health records on healthcare quality: a systematic review and meta-analysis Eur J Public Health 26(1) 60–64 https://doi.org/10.1093/eurpub/ckv122
31. Bamodu OA and Chung CC (2024) Cancer care disparities: overcoming barriers to cancer control in low-and middle-income countries JCO Global Oncol 10 e2300439 https://doi.org/10.1200/GO.23.00439
32. Kabukye JK, Kakungulu E, and de Keizer N, et al Digital health in oncology in Africa: a scoping review and cross-sectional survey Int J Med Inform 158 104659 PMID: 34929545
33. Ali O, Abdelbaki W, and Shrestha A, et al (2023) A systematic literature review of artificial intelligence in the healthcare sector: benefits, challenges, methodologies, and functionalities J Innov Knowl 8(1) 100333 https://doi.org/10.1016/j.jik.2023.100333
34. Gu D, Khan S, and Khan IU, et al (2021) Assessing the adoption of e-health technology in a developing country: an extension of the UTAUT model Sage Open 11(3) 21582440211027565 https://doi.org/10.1177/21582440211027565
35. Bagherian H and Sattari M (2022) Health information system in developing countries: a review on the challenges and causes of success and failure Med J Islam Repub Iran 36 111 PMID: 36447547 PMCID: 9700424
36. Barros Leal Farias D (2024) Unpacking the ‘developing’country classification: origins and hierarchies Rev Int Polit Econ 31(2) 651–673 https://doi.org/10.1080/09692290.2023.2246975
37. Syzdykova A, Malta A, and Zolfo M, et al (2017) Open-source electronic health record systems for low-resource settings: systematic review JMIR Med Inform 5(4) e8131 https://doi.org/10.2196/medinform.8131
38. Bervell B and Al-Samarraie H (2019) A comparative review of mobile health and electronic health utilization in sub-Saharan African countries Soc Sci Med 232 1–16 https://doi.org/10.1016/j.socscimed.2019.04.024 PMID: 31035241
39. Moher D, Shamseer L, and Clarke M, et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement Syst Rev 4(1) 1 https://doi.org/10.1186/2046-4053-4-1 PMID: 25554246 PMCID: 4320440
40. Page MJ, McKenzie JE, and Bossuyt PM, et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews bmj 372 PMID: 33782057 PMCID: 8005924
41. Macdonald M, Misener RM, and Weeks L, et al (2016) Covidence vs Excel for the title and abstract review stage of a systematic review JBI Evid Implement 14(4) 200–201
42. Moher D, Liberati A, and Tetzlaff J, et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement Bmj 339 https://doi.org/10.1136/bmj.b2535
43. Minozzi S, Cinquini M, and Gianola S, et al (2020) The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application J Clin Epidemiol 126 37–44 https://doi.org/10.1016/j.jclinepi.2020.06.015 PMID: 32562833
44. Sterne JA, Hernán MA, and Reeves BC, et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions bmj 355
45. Shea BJ, Grimshaw JM, and Wells GA, et al (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews BMC Med Res Methodol 7(1) 10 https://doi.org/10.1186/1471-2288-7-10 PMID: 17302989 PMCID: 1810543
46. Pollock M, Fernandes RM, and Hartling L (2017) Evaluation of AMSTAR to assess the methodological quality of systematic reviews in overviews of reviews of healthcare interventions BMC Med Res Methodol 17(1) 48 https://doi.org/10.1186/s12874-017-0325-5 PMID: 28335734 PMCID: 5364717
Supplementary
Supplementary file 1. PRISMA-P 2015 checklist: recommended items to address in a systematic review protocol*.