Anal cancer impact among people with HIV infection – a matched cohort study
Amanda Acioli de Almeida Robatto1,a, Erika Andrade Rocha1,b, Renata Colombo Bonadio1,2,c, Denis Artico Galhera3,d, Carolina Teixeira Muratori1,e, Admir Andre Belo Bueno1,f, Abraão Ferreira Lopes Dornellas1,g, Luciana Bastos Valente Alban1,h, Carolina Ribeiro Victor1,2,i, Maria Ignez Freitas Melro Braghiroli1,2,j, Marília Polo Mingueti e Silva1,2,k, Camila Soares Araujo1,l, Carlos Frederico Sparapan Marques1,m, Caio Sergio Rizkallah Nahas1,n, Karim Yaqub Ibrahim1,o, André Tsin Chih Chen1,p, Paulo Marcelo Gehm Hoff1,2,q and Camila Motta Venchiarutti Moniz1,2,r
1ICESP – Instituto do Câncer do Estado de São Paulo, Dr. Arnaldo Avenue, Sao Paulo 01246-000, Brazil
2IDOR – Instituto D’Or de Pesquisa e Ensino, Sao Paulo 01401-002, Brazil
3Universidade São Caetano do Sul, São Caetano do Sul 09521-160, Brazil
ahttps://orcid.org/0000-0002-0255-0765
bhttps://orcid.org/0009-0008-8275-0345
chttps://orcid.org/0000-0001-5818-922X
dhttps://orcid.org/0000-0002-6534-3755
ehttps://orcid.org/0009-0007-1567-8665
fhttps://orcid.org/0009-0008-9140-2764
ghttps://orcid.org/0000-0003-3634-8503
hhttps://orcid.org/0009-0004-0147-8062
ihttps://orcid.org/0000-0001-8179-4639
jhttps://orcid.org/0000-0001-6366-8786
khttps://orcid.org/0009-0002-9681-3374
lhttps://orcid.org/0000-0003-4675-3369
mhttps://orcid.org/0000-0003-4293-6301
nhttps://orcid.org/0000-0001-8036-512X
ohttps://orcid.org/0000-0002-5074-3860
phttps://orcid.org/0000-0001-5183-1048
qhttps://orcid.org/0000-0002-0065-2194
rhttps://orcid.org/0000-0002-1182-4764
Abstract
Background: Pivotal studies with curative chemoradiation (CRT) in anal cancer did not include HIV-positive (HIV+) patients. HIV status impact remains unknown in this scenario.
Methods: In this retrospective matched cohort study, electronic medical records were reviewed at Sao Paulo State Cancer Institute between 2010 and 2021 patients with anal cancer T1-4 N0-1 M0 by AJCCVIII were selected. For each HIV+ patient, one or two HIV-negative (HIV-) cases were matched by age, stage (T, N) and ECOG. The primary endpoint was OS, estimated using Kaplan-Meir and compared with the log-rank test.
Results: 122 patients were selected, 45 being HIV+. The median follow-up was 37 months. Most patients, n = 119 (98%), received concomitant CRT and had ECOG 0/1 (n = 116, 95%). Stage III corresponded to 69% of the patients (n = 85). Positive nodes were detected in 76 patients (62%). No difference was observed in complete clinical response (cCR) post-CRT (68% in HIV+ versus 63% in HIV-; p = 0.6). Median recurrence-free survival (RFS) was not reached; 3-year RFS rates were 60.7% in HIV+ versus 63.1% in HIV- [hazard ratio (HR) 1.20, 95% CI 0.66–2.17, p = 0.538]. Median OS was not reached; 3-year OS was 66.4% HIV+ versus 72.2% in HIV- (HR 1.23, 95% CI 0.61–2.47, p = 0.546). HIV+ pts presented significantly more hospital admissions due to toxicity, 30% (n = 12/40) versus 13% (n = 10/74) (p = 0.049). No difference between groups was found for colostomy (p = 0.69) and salvage surgery (p = 1).
Conclusion: Anal carcinoma HIV+ patients treated with CRT presented similar cCR, RFS and OS compared with HIV- patients. Optimal therapy should be attempted in the HIV+ population; however, close clinical monitoring due to higher hospital admission is required.
Keywords: anal carcinoma, squamous cell carcinoma of the anus, HIV, chemoradiation, CRT
Correspondence to: Amanda Acioli de Almeida Robatto
Email: amanda.acioli@hc.fm.usp.br
Published: 03/06/2025
Received: 19/11/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Anal cancer is a rare disease, accounting for just 0.5% of all new cancer cases [1]. Still, its age-adjusted death rate has been steadily rising, averaging an increase of 5.1% annually from 2013 to 2022 [1]. The 5-year relative survival rates for squamous cell carcinoma of the anus (SCCA) vary significantly depending on the disease stage: 84.5% for localised disease, 68.2% for regional disease and 36.3% for distant metastases [1]. SCCA is the most common subtype of anal cancer, representing approximately 90% of cases and is strongly associated with immunosuppression and human papillomavirus (HPV) infection [2].
People living with HIV/AIDS are at an increased risk for both in situ and invasive HPV-associated cancers, including anal cancer. The HIV population is 19 times more likely to develop anal cancer compared to the general population, with men at a higher risk (hazard ratio [HR], 20.73; 95% confidence interval [CI], 15.60–27.56) and women are also significantly at risk (HR, 12.88; 95% CI, 8.69–19.07) (3). Despite the high incidence of SCCA in HIV-positive (HIV+) patients, this population is often underrepresented in key prospective clinical trials on chemoradiotherapy (CRT) and the impact of CRT in this group remains poorly understood. This study aimed to evaluate differences in overall survival (OS), recurrence-free survival (RFS), complete clinical response (cCR), toxicity, colostomy and salvage surgery between HIV+ and HIV-negative (HIV-) patients with localised SCCA undergoing standard-of-care curative treatment.
Materials and Methods
This is a retrospective matched cohort study. We reviewed electronic medical records from 2010 to 2021 at the Instituto do Câncer do Estado de São Paulo, Brazil.
Ethical consideration
This study followed the guidelines of the Declaration of Helsinki. It was approved by the ethics committee and the institutional review board of Instituto do Câncer do Estado de São Paulo, Brazil. Due to the retrospective nature of the study, the requirement for informed consent was waived.
Patients
Inclusion criteria were histologically confirmed invasive SCCA and stage I-III by AJCC VIII Edition treated with curative intent.
Endpoints
The primary endpoint was OS. The secondary endpoints were RFS, cCR, toxicities, colostomy and salvage surgery.
Statistical analysis
HIV+ patients were matched using the R package ‘MatchIt’ with one or two non-metastatic HIV- cases, based on age, stage (T, N) and ECOG performance status, as these were considered the primary matching criteria.
The OS, RFS and cCR were estimated using Kaplan-Meir and log-rank tests. Toxicities were evaluated in patients who received CRT. To handle missing data in toxicities analyses, we excluded patients with no information about specific variables. A maximum of five patients were excluded from each group, as detailed in the supplementary material (Supplemental Tables 1 and 2). The p-value for toxicities during CRT was evaluated using the chi-square test.
The comparison between HIV+ and HIV- patients for colostomy and salvage surgery were analysed using Fisher’s exact test.
Results
We included 122 patients, 45 HIV+ patients, with a median follow-up of 37 months. The median age was 59 years in the HIV- group and 48 in the HIV+ group. We observed a high prevalence of males in the HIV+ group patients (n = 31, 68.8%) and females (n = 62, 80.5%) in the HIV- group. Stage III was the most frequent in both groups; most T4 (n = 41, 33%) and T3 tumours (n = 36, 29%). Positive nodes were detected in n = 76 (62%) patients (Table 1). Most patients (n = 119, 98%) received concomitant CRT as treatment with curative intent and had ECOG 0/1 (n = 116, 95%). Definitive concomitant regimens included mitomycin C (MMC) plus infusional 5-Fluorouracil (5-FU) (n = 37; 31%), cisplatin (CDDP) plus capecitabine (n = 26; 21,8%) and CDDP plus infusional 5-FU (n = 35; 29%). Patients received a median of 54 Gy in 30 fractions in the primary tumour and 45 Gy in elective lymph node drainage. Three-dimensional conformal radiotherapy, intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT) were used in 23%, 47% and 29% of patients, respectively.
Table 1. Characteristics of the population.
Three-year OS rates were 66.4% in HIV+ patients versus 72.2% in HIV- patients (HR 1.23, 95% CI 0.61–2.47, p = 0.546). Three-year RFS rates were 60.7% in the HIV+ group versus 63.1% HIV- group (HR 1.20, 95% CI 0.66–2.17, p = 0.538). Median OS and RFS were not reached (Figures 1 and 2).
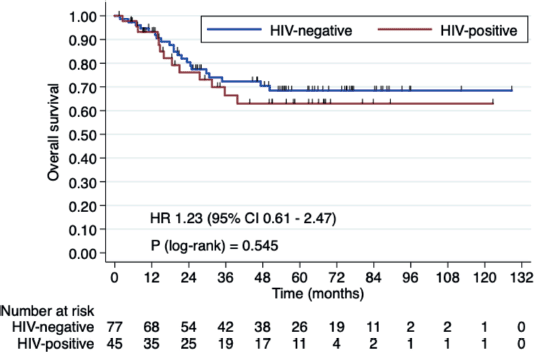
Figure 1. OS in HIV+ patients versus HIV- patients.
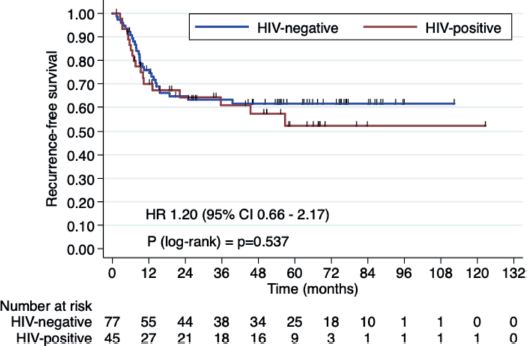
Figure 2. RFS in HIV+ patients versus HIV- patients.
cCR at 6 months post-CRT was 68% in HIV+ patients and 63% in HIV- patients (p = 0.6) (Table 2).
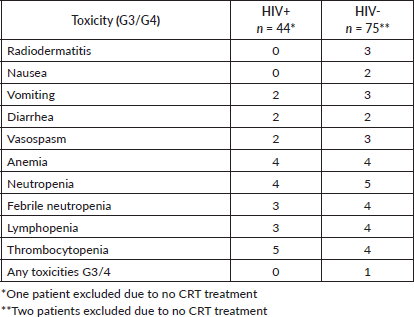
HIV+ patients had significantly more hospital admissions due to toxicity (n = 12, 30%) than HIV- (n = 10, 13.8%) (p = 0.049) (Table 3). The main toxicities were radiodermatitis in both groups, 45% in HIV+ patients and 40% in HIV- patients and lymphopenia, 50% in HIV+ patients and 43% in HIV- patients (Table 4).
Table 2. Outcomes of CRT in HIV+ and HIV- patients.
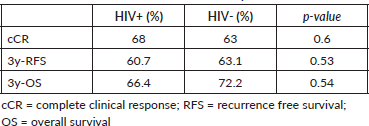
Table 3. Toxicities during CRT.
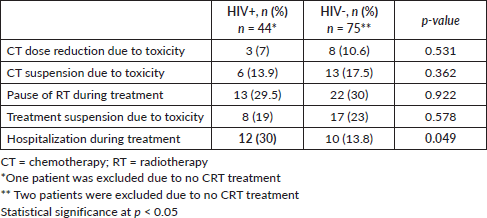
Table 4. Adverse events of CRT.
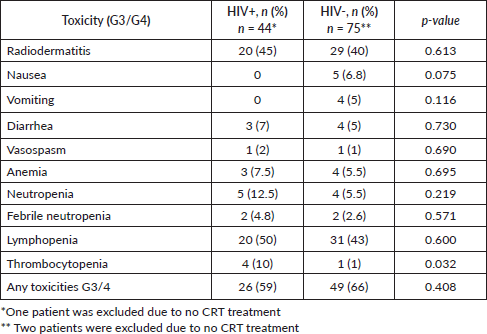
Table 5. Colostomy and salvage surgery HIV+ versus HIV- patients.

Some patients required a colostomy or salvage surgery before, during or immediately after CRT. When analyzing only the patients who underwent CRT, colostomy was performed in 29.5% of HIV+ patients and 33.3% of HIV- patients (Table 5). Salvage surgery was performed in 11.3% of HIV+ patients and 10.6% of HIV- patients. No statistically significant differences were found when comparing patients based on HIV status. The odds ratio (OR) for colostomy was 0.84 (95% CI, 0.34–2.0; p = 0.69), and for salvage surgery, it was 1.05 (95% CI, 0.25–3.97; p = 1).
Discussion
SCCA is a rare cancer, representing just 0.5% of all new cancer cases, according to the SEER database. Early diagnosis and timely treatment are crucial for better outcomes since 84.5% of patients with localised disease and 68.2% with regional disease are alive in 5 years [1]. Preventable risk factors are associated with SCCA, with HPV being the most prominent, particularly when combined with HIV infection [3]. As a result, offering HIV testing to patients newly diagnosed with SCCA is strongly recommended [4].
The standard treatment for non-metastatic SCCA involves CRT with a fluoropyrimidine, such as 5-fluorouracil and an MMC regimen, which yields a 67% OS rate at 5 years [5]. An alternative treatment option is CDDP combined with fluoropyrimidine, with no significant differences in complete response rates or progression-free survival (PFS) between the CDDP and MMC groups [6]. However, HIV+ patients were not included in these phase III clinical trials, so data on key endpoints such as OS, PFS and toxicity in this population remains unavailable.
Contemporary studies are increasingly focusing on the HIV+ population. In a prospective cohort study that included 12% HIV+ patients treated with a full dose of MMC and fluoropyrimidine, HIV positivity was associated with a worse response rate (OR 5.72; 95% CI 2.5–13.0; p < 0.001). The 5-year PFS and OS rates were 63.3% and 76.4%, respectively, with a median follow-up of 66 months. Multivariate analysis revealed that the absence of complete response at 6 months (HR 3.36, p = 0.007, 95% CI 1.39–8.09) was associated with inferior OS [7]. In a Phase II prospective study evaluating MMC plus capecitabine during CRT, 9.3% of the participants were HIV+, with an OS of 97.7% at a median follow-up of 23.1 months; however, there was no comparison between HIV+ and HIV- patients [8].
In our current study, we aimed to assess whether there is a significant difference in outcomes between HIV+ and HIV- patients undergoing CRT. Our findings showed that the 3-year OS rates were 66.4% for HIV+ patients versus 72.2% for HIV- patients (HR 1.23, 95% CI 0.61–2.47, p = 0.546). The 3-year RFS rates were 60.7% for the HIV+ group and 63.1% for the HIV- group (HR 1.20, 95% CI 0.66–2.17, p = 0.538). Median OS and RFS were not reached. These results support the idea that HIV+ status does not significantly affect CRT outcomes (OS and RFS) when properly managed.
Another important consideration is that HIV+ patients in the era of modern antiretroviral therapy can achieve adequate viral load suppression and maintain healthy CD4 counts. However, due to the absence of established guidelines and limited recent data on CRT tolerance and outcomes in the HIV+ population, adjustments to radiotherapy and chemotherapy doses are still made at the discretion of the treating physician.
Regarding toxicities, three retrospective studies involving HIV-infected patients during CRT reported relevant data. One of the most common toxicities was grades 3 and 4 dermatitis, which ranged from 23% to 50%. Grades 3 and 4 hematological and gastrointestinal toxicities were also frequently observed, with rates ranging from 30% to 38% and 15% to 30%, respectively [9, 10, 11]. Additionally, neutropenia and lymphopenia were commonly observed in HIV+ patients undergoing CRT [12]. A small retrospective cohort also identified a pretreatment CD4 count below 200 as a factor associated with poorer anal cancer control and increased treatment-related morbidity [13]. In this cohort, HIV+ patients more frequently experienced lymphopenia (50%) and radiodermatitis (45%) as grade 3 and 4 toxicities. Furthermore, they had significantly higher rates of hospitalisation due to toxicity (30%, n = 12/40) compared to HIV- patients (13.8%, n = 10/72; p = 0.049).
Quality of life (QoL) is another crucial outcome for patients with anal cancer, particularly for those who are HIV+. Several factors influence their QoL, including the impact of a colostomy, cancer treatment and HIV itself. Treatment for anal cancer, typically involving chemoradiotherapy, can lead to significant side effects such as bowel dysfunction, fatigue and sexual dysfunction, which may persist long-term and significantly affect QoL [14, 15, 16]. The addition of a colostomy further complicates these issues, often resulting in body image concerns, challenges with social functioning and sexual health difficulties [17]. These physical and psychosocial burdens can lead to a marked decline in overall QoL. Although studies specifically focusing on HIV+ patients with anal cancer and colostomies are limited, related research indicates that their QoL may be further compromised compared to HIV- patients. The existing literature underscores the need for more targeted research to better understand the unique QoL outcomes in this population and address both the physical and psychosocial factors critical to improving their overall well-being [17, 18].
As a retrospective cohort study, our data regarding QoL were limited; however, the use of colostomy and salvage surgery was evaluated. Among HIV- patients who underwent CRT, 33.3% required a colostomy and 10.6% underwent salvage surgery. Among HIV+ patients, 29.5% required a colostomy and 11.3% underwent salvage surgery. No statistically significant difference was found when comparing HIV+ to HIV- groups: the OR for colostomy was 0.84 (95% CI 0.34–2.0; p = 0.69) and for salvage surgery was 1.05 (95% CI 0.25–3.97; p = 1).
Our study’s retrospective design has inherent limitations, including small sample size, missing data due to variability in patient registries, lack of patient stratification by CD4 count and specific CRT regimens. Patients were not stratified by CD4 count due to its unavailability for all subjects. Additionally, stratification by treatment regimen was not performed, as we did not anticipate any imbalance between the groups.
However, since most patients received standard CRT, our findings offer valuable insights into the safety and efficacy of CRT in HIV+ individuals. Our data suggest that HIV+ patients with good performance status (ECOG 0–1) can safely undergo standard CRT. However, due to their increased risk of toxicity, vigilant monitoring and timely treatment adjustments are crucial. Efforts are underway to include the HIV+ population in prospective studies, such as the Phase III POD1UM-303/InterAACT 2 trial [19]. However, this study focused on metastatic SCCA. Future prospective studies involving patients undergoing CRT for localised SCCA should aim to identify optimal treatment strategies for this population, incorporating immunological parameters like CD4 count and HIV viral load to refine treatment guidelines.
Conclusion
In this matched cohort study, HIV+ patients with localised anal carcinoma and good performance status who were treated with standard CRT showed similar outcomes in terms of OS, RFS, cCR, colostomy and salvage surgery compared to HIV- patients. Therefore, optimal therapy should be pursued for the HIV+ population, with close clinical monitoring due to the significantly higher risk of hospital admissions related to treatment toxicity in this group.
It is crucial to include HIV+ patients in ongoing prospective clinical trials, particularly those involving novel targeted therapies with expected lower bone marrow toxicity, to provide future evidence on response rates and treatment tolerance within this population.
Acknowledgment
We thank the Institute of Cancer of Sao Paulo (ICESP) for caring for the study participants and making this study possible.
Conflicts of interest and funding declaration
The authors declare no conflicts of interest related to the development of this work. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Prior presentation
Presented as Poster at European Society of Medical Oncology (ESMO) Congress 2022, September 9–14, 2022.
References
1. Cancer Stat Facts: Anal Cancer [https://seer.cancer.gov/statfacts/html/anus.html] Date accessed: 21/02/25
2. Saraiya M, Unger ER, and Thompson TD, et al (2015) US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines J Natl Cancer Inst 107(6) djv086
3. Michaud JM, Zhang T, and Shireman TI, et al (2020) Hazard of cervical, oropharyngeal, and anal cancers in HIV-infected and HIV-uninfected medicaid beneficiaries Cancer Epidemiol Biomarkers Prev 7 1447–1457 https://doi.org/10.1158/1055-9965.EPI-20-0281
4. Rao S, Guren MG, and Khan K, et al (2021) Anal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up Ann Oncol 9 1087–1100 https://doi.org/10.1016/j.annonc.2021.06.015
5. Leichman L, Nigro N, and Vaitkevicius VK, et al (1985) Cancer of the anal canal. Model for preoperative adjuvant combined modality therapy Am J Med 2 211–215 https://doi.org/10.1016/0002-9343(85)90428-0
6. James RD, Glynne-Jones R, and Meadows HM, et al (2013) Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial Lancet Oncol 6 516–524 https://doi.org/10.1016/S1470-2045(13)70086-X
7. Moniz CMV, Riechelmann RP, and Oliveira SCR, et al (2021) A prospective cohort study of biomarkers in squamous cell carcinoma of the anal canal (SCCAC) and their influence on treatment outcomes J Cancer 23 7018–7025 https://doi.org/10.7150/jca.57678
8. Oliveira SC, Moniz CM, and Riechelmann R, et al (2016) Phase II study of capecitabine in substitution of 5-FU in the chemoradiotherapy regimen for patients with localized squamous cell carcinoma of the anal canal J Gastrointest Cancer 1 75–81 https://doi.org/10.1007/s12029-015-9790-4
9. Chadha M, Rosenblatt EA, and Malamud S, et al (1994) Squamous-cell carcinoma of the anus in HIV-positive patients Dis Colon Rectum 9 861–865 https://doi.org/10.1007/BF02052589
10. Kim JH, Sarani B, and Orkin BA, et al (2001) HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients Dis Colon Rectum 10 1496–1502 https://doi.org/10.1007/BF02234605
11. Holland JM and Swift PS (1994) Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy Radiology 1 251–254 https://doi.org/10.1148/radiology.193.1.8090901
12. Vendrell I, Ferreira AR, and Abrunhosa-Branquinho AN, et al (2018) Chemoradiotherapy completion and neutropenia risk in HIV patients with cervical cancer Medicine (Baltimore) 30 e11592 https://doi.org/10.1097/MD.0000000000011592
13. Hoffman R, Welton ML, and Klencke B, et al (1999) The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer Int J Radiat Oncol Biol Phys 1 127–131 https://doi.org/10.1016/S0360-3016(98)00528-8
14. Sauter C, Peeken JC, and Borm K, et al (2022) Quality of life in patients treated with radiochemotherapy for primary diagnosis of anal cancer Sci Rep 1 4416 https://doi.org/10.1038/s41598-022-08525-1
15. Sterner A, Derwinger K, and Staff C, et al (2019) Quality of life in patients treated for anal carcinoma- a systematic literature review Int J Colorectal Dis 9 1517–1528 https://doi.org/10.1007/s00384-019-03342-x
16. Das P, Cantor SB, and Parker CL, et al (2010) Long-term quality of life after radiotherapy for the treatment of anal cancer Cancer 4 822–829 https://doi.org/10.1002/cncr.24906
17. Robitaille S, Maalouf MF, and Penta R, et al (2023) The impact of restorative proctectomy versus permanent colostomy on health-related quality of life after rectal cancer surgery using the patient-generated index Surgery 4 813–818 https://doi.org/10.1016/j.surg.2023.06.033
18. Mauro GP, da Conceição Vasconcelos KGM, and Carvalho HA (2021) Quality of life and sexual function of men who have sex with men treated for anal cancer: a prospective trial of a neglected population J Sex Med 8 1461–1466 https://doi.org/10.1016/j.jsxm.2021.05.015
19. Rao S, Samalin-Scalzi E, and Evesque L, et al (2024) POD1UM-303/InterAACT 2: Phase III study of retifanlimab with carboplatin-paclitaxel (c-p) in patients (Pts) with inoperable locally recurrent or metastatic squamous cell carcinoma of the anal canal (SCAC) not previously treated with systemic chemotherapy (Chemo) Ann Oncol Suppl 35 S1217 https://doi.org/10.1016/j.annonc.2024.08.2262
Supplementry
Supplemental Table 1. Excluded patients from analysis of toxicities during CRT per group due to missing data.

Supplemental Table 2. Excluded patients from analysis of adverse events of CRT per group due to missing data.