Locally advanced and metastatic esophageal squamous cell carcinoma in Morocco: from diagnosis to treatment
Anass Baladi1, Hassan Abdelilah Tafenzi1,2, Fatim-Zahra Megzar1, Ibrahima Kalil Cisse1, Othmane Zouiten1, Leila Afani1, Ismail Essaadi2,3, Mohammed El Fadli1 and Rhizlane Belbaraka1,2
1Department of Medical Oncology, Mohammed VI University Hospital of Marrakech, Marrakesh 40000, Morocco
2Biosciences and Health Laboratory, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Morocco
3Medical Oncology Department, Avicenna Military Hospital of Marrakech, Marrakech 40000, Morocco
Abstract
Background: Esophageal squamous cell carcinoma (ESCC) is the most common subtype of esophageal cancer (EC) worldwide, with significant geographic variability in its incidence and outcomes. This study aims to analyse the characteristics of Moroccan ESCC patients, identify independent prognostic factors for mortality and assess access to surgery, chemotherapy, radiotherapy (RT) and targeted therapies.
Methods: This retrospective study analysed data from the Marrakesh-Safi regional cancer registry. Between 2014 and 2019, a total of 78 patients were histologically confirmed to have locally advanced or metastatic ESCC. Demographic, clinical and treatment data were evaluated to determine prognostic factors for overall survival (OS) using Kaplan–Meier and multivariate Cox regression analyses.
Results: The median age was 56 years (IQR: 48–66), with a slight female predominance in stage III (59%). Dysphagia was the most frequent symptom (92%), and the thoracic esophagus was the most common tumour site (53%). Performance status was significantly worse in stage IV (31% with PS 4, p < 0.001). Chemotherapy was administered to 72% of patients, with cisplatin being the most used drug. RT was more common in stage III (57% versus 33%, p = 0.035), while surgery was rare (2 cases). Multivariate analysis identified performance status as a key prognostic factor (HR = 27.2, p = 0.015), while RT significantly reduced mortality risk (HR = 0.07, p = 0.038). Stage III patients had a median OS of 46 months, with 1- and 3-year OS rates of 84% and 78%, respectively. In contrast, stage IV patients had a median OS of 8.6 months, with 1-year and 3-year OS rates of 34% and 22%, respectively.
Conclusion: Patients with locally advanced or metastatic EC face poor survival outcomes. RT and performance status are key factors that significantly influence prognosis. These findings underscore the urgent need for early detection, enhanced access to multimodal treatments and improved healthcare infrastructure to improve survival outcomes.
Keywords: esophageal cancer, esophageal squamous cell carcinoma, prognostic factors
Correspondence to: Anass Baladi
Email: anassbaladi2020@gmail.com
Published: 01/04/2025
Received: 20/08/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Esophageal cancer (EC) remains a significant global health concern, with varying trends in its incidence. According to a comprehensive analysis reported by Mazidimoradi et al [1], there were over 600,000 new cases of EC in 2020, with esophageal squamous cell carcinoma (ESCC) being the predominant subtype, especially in Eastern Asia, South Central Asia and Sub-Saharan Africa. Overall, the age-standardised rates for EC, including both incidence and mortality, have been declining globally since 1990, with variations across different regions and countries [2]. These statistics underscore the importance of continued research and targeted prevention strategies to address the burden of EC on a global scale.
In North Africa, and particularly in Morocco, reliable epidemiological data on EC remains scarce. However, the World Health Organisation highlights the growing burden of cancer in Morocco, where EC in Morocco ranks 23rd in incidence among all cancer types and 17th in terms of cancer-related deaths. The lifetime risk of death from EC is estimated to be around 1.1% [3]. According to the latest cancer statistics, EC, particularly ESCC, is linked to various common risk factors globally. Studies have identified key risk factors such as tobacco and alcohol use, polycyclic aromatic hydrocarbon exposure, hot food and beverage consumption, poor oral health and a diet low in fruits and vegetables [4–6]. Understanding these diverse risk factors is crucial for developing targeted prevention strategies and interventions to reduce the global burden of EC effectively [7]. For instance, although the 5-year survival rate for distant ESCC is barely 5%, it is 46.4% for localised ESCC [8]. Its poor prognosis is mainly based on the lack of early detection of precancerous lesions due to the late consultation of patients with the first symptom. Unfortunately, EC is most often discovered late, and most often, at the time of diagnosis, the cancer is already advanced and unresectable, given the anatomical location of the esophagus and its contiguity with vital organs [9]. To manage ESCC patients appropriately and increase their overall survival (OS) probability, an early and precise identification of the disease is crucial. EC is frequently found after it is already advanced, necessitating very invasive therapies such as surgical resection and chemoradiotherapy [10].
Due to a lack of information regarding North African ESCC, in this study, we brought to light the actual situation concerning patients with ESCC from diagnosis to treatment, all by detailing features, the independent prognostic factors related to OS and the accessibility of treatments from surgery, chemotherapy, radiotherapy (RT) and targeted therapies, to provide insights and the most recent data.
Materials and methods
Patients diagnosed with ESCC between 2014 and 2019 were identified from the medical oncology department of Mohammed VI University Hospital in Marrakech, based on their stage at diagnosis. The date of occurrence was considered the day of the biopsy. Follow-up data for each patient were compiled using the most recent medical records, including clinical examinations and recent computed tomography (CT) assessments. In cases where patients were lost to follow-up, they were contacted using their phone numbers. Only patients who met the following criteria were eligible for this study: those histologically diagnosed with ESCC as a primary malignancy confirmed either by biopsy between 2014 and 2019 and those who had clinically locally advanced or metastatic disease. Patients were excluded if they had documented clinical T0 (no evidence of a primary tumour), Tis (high-grade dysplasia) or if they were diagnosed at early stages (I-IIb). RT and chemotherapy, with or without surgery, are recognised as part of a bi- or tri-modal curative-intention approach for EC, in line with global standards. Survival was defined as the period from the patient’s enrollment date to the date of death. For patients lost to follow-up, we made efforts to contact families to ascertain the date of death, thereby ensuring the accuracy of our survival data. Data for patients who were still living and had not experienced a relapse were censored as of the last known follow-up appointment date.
Patient characteristics for ESCC were systematically collected, including sex (male, female), age at diagnosis and smoking status (yes, no), as well as initial symptoms (dysphagia, dyspnea, vomiting, epigastric pain and general condition impairment). Histopathological data comprised the anatomical location, tumour differentiation and macroscopic tumour features (ulcerative-budding and stenosing, stenosing, ulcer-infiltrative, infiltrative, ulcer-budding and non-specific appearance). Tumour invasion was assessed using CT scans by evaluating lymphadenopathy in regions such as the subcarinal, mediastinal, jugular-carotid and perigastric nodes. The scans also identified tumour contact with adjacent structures, including the descending aorta and the para-laryngeal region. The PS was evaluated using the Eastern Cooperative Oncology Group Performance Status (ECOG PS), and clinical T, N and M categories and cancer stage at diagnosis were determined according to the 7th edition of the American Joint Committee on Cancer (AJCC). Metastatic sites (lung, liver, bone and adrenal) were also recorded at diagnosis. In cases where specific T, N and M details were unavailable, the overall stage according to the 7th edition of AJCC, as documented in the medical records, was used, reflecting the clinician’s synthesis of available diagnostic data. We documented the administration of various treatments for ESCC, specifying whether patients received surgery, RT or chemotherapy.
RT is crucial for treating ESCC. Neoadjuvant chemoradiotherapy improves survival and surgical outcomes. For patients who cannot undergo surgery, definitive RT, with or without chemotherapy, is an option. Palliative RT effectively relieves dysphagia and pain. For chemotherapy, we detailed the specific regimens used, which included 5-fluorouracil (5-FU), Folfox (a combination of Oxaliplatin, 5-FU and Leucovorin), Xelox (a combination of Capecitabine and Oxaliplatin), Xeloda (Capecitabine), Cisplatin and Docetaxel. The treatment administration was recorded as either ‘Yes’ if the patient received the treatment or ‘No’ if they did not.
Categorical variables were expressed as percentages, while continuous variables such as age were expressed as medians and quartiles. Cox proportional hazard regression analyses were used to identify the independent factors related to OS in patients diagnosed with ESCC. The selection of the most appropriate statistical test depends on the specific characteristics of the variable under consideration. Tests such as the Wilcoxon rank-sum test, Pearson’s chi-squared test and Fisher’s exact test are chosen based on the unique requirements of the data. Additionally, the Kaplan–Meier method was used to estimate all time-to-event distributions based on prognostic variables selected from the multivariate Cox proportional hazard regression analysis and compared by the log-rank test. A p-value of less than 0.05 was considered statistically significant. The ‘survival’ and ‘survminer’ packages in R software were used for all statistical analyses.
Results
Description
Over the 5 years from 2014 to 2019, the study included 78 patients diagnosed with ESCC, divided into stage III (n = 42) and stage IV (n = 36). The population in our study represents a regional subset of ESCC patients treated specifically at the Mohammed VI University Hospital in the Marrakesh-Safi region of Morocco. The median follow-up period was approximately 8.5 months (0 to 48.9 months). The demographic, clinical, pathological and therapeutic characteristics of the patients with both locally advanced and metastatic ESCC are summarised in Table 1.
The median age at diagnosis of the patients was 56 years (IQR: 48–66), with no significant difference between stages. Females were slightly predominant (59%) in stage III (p = 0.14). Dysphagia was the most common symptom, affecting 92% of patients. The most frequent tumour location was the thoracic esophagus, observed in 40% of patients and the predominant macroscopic appearance was stenosing (48%). PS was significantly worse in stage IV, with 31% of patients having a PS of 4 (p < 0.001), indicating a marked deterioration in physical condition at advanced stages of the disease. Among the observed metastases, lung metastases were the most common (24%), followed by liver (9%), bone (7.7%) and adrenal metastases (2.6%). No brain metastases or other distant metastatic sites outside the recorded categories were observed in our cohort.
Among the 78 patients in the study, 22 (28%) did not receive chemotherapy, comprising 9 patients with stage III and 13 with stage IV. Of the 56 patients (72%) who received chemotherapy, 13 with stage III underwent neoadjuvant chemotherapy, 10 received concurrent chemotherapy and 10 received palliative chemotherapy. In contrast, 23 patients with stage IV were treated with palliative chemotherapy. No significant difference in the overall administration of chemotherapy was observed between stage III and stage IV patients (p = 0.15). The most frequently used chemotherapy agents were cisplatin and 5FU, with Xeloda and Xelox used less commonly. RT was more commonly administered to patients in stage III, with 57% receiving localised RT, compared to 33% of stage IV patients who received metastatic RT. Stage III patients received a median of 15 RT sessions with 30 total fractions, while stage IV patients received a median of 10 sessions with 20 total fractions. Surgical intervention was rare, with only 2 out of 78 patients undergoing surgery – one diagnosed at stage III and one at stage IV.
Survival analysis
Multivariate Cox regression analysis identified independent prognostic factors associated with survival. PS emerged as a key factor, with patients having a PS of 3 showing a significantly increased mortality risk (HR = 27.2, 95% CI: 1.87–394, p = 0.015), making PS a strong independent predictor of poor prognosis. Additionally, RT proved to be an important protective factor, significantly reducing the risk of mortality (HR = 0.07, 95% CI: 0.01–0.86, p = 0.038). In contrast, chemotherapy was not significantly associated with improved survival after adjusting for other variables (HR = 0.48, 95% CI: 0.07–3.36, p = 0.5) (Table 2).
For patients with stage III, the median OS was 46 months (95% CI, 46 to not estimable). The 1-year OS rate was 84% (95% CI, 72% to 97%), and the 3-year OS rate was 78% (95% CI, 64% to 95%). In contrast, the median OS for patients with stage IV was 8.6 months (95% CI, 3.3 to 22). The 1-year OS rate was 34% (95% CI, 21% to 55%), and the 3-year OS rate was 22% (95% CI, 11% to 48%) (Figure 1).
Table 1. Clinical characteristics of ESCC patients by stage at diagnosis (Stage III versus IV) at Mohammed VI Oncological Center, Marrakech (2014–2019).
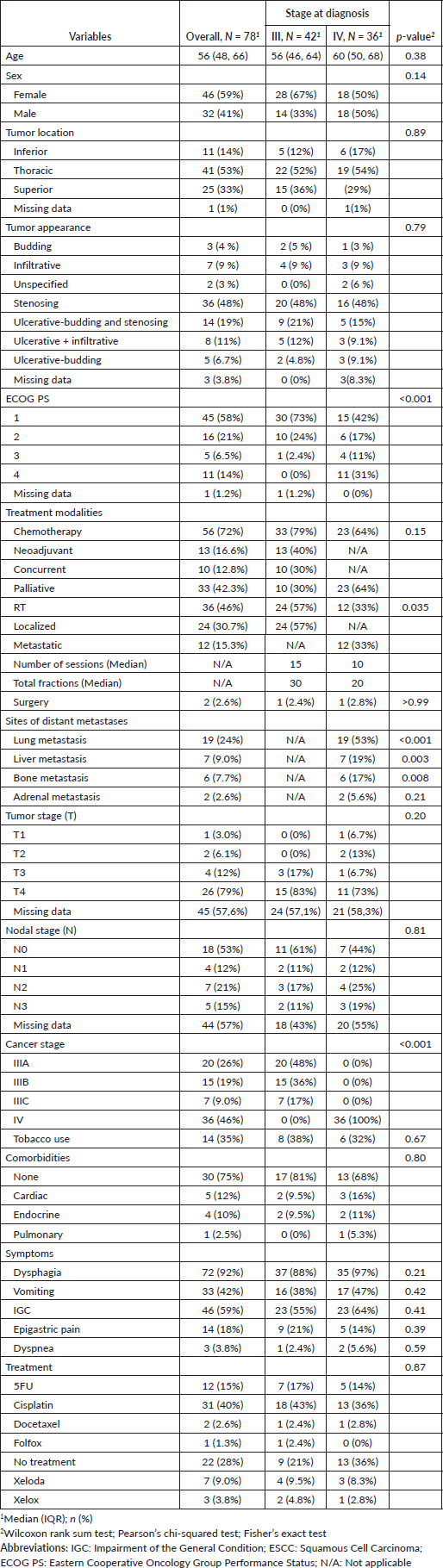
Table 2. Univariate and multivariate cox regression analysis of ESCC patients at Mohammed VI Oncological Center, Marrakech (2014-2019).
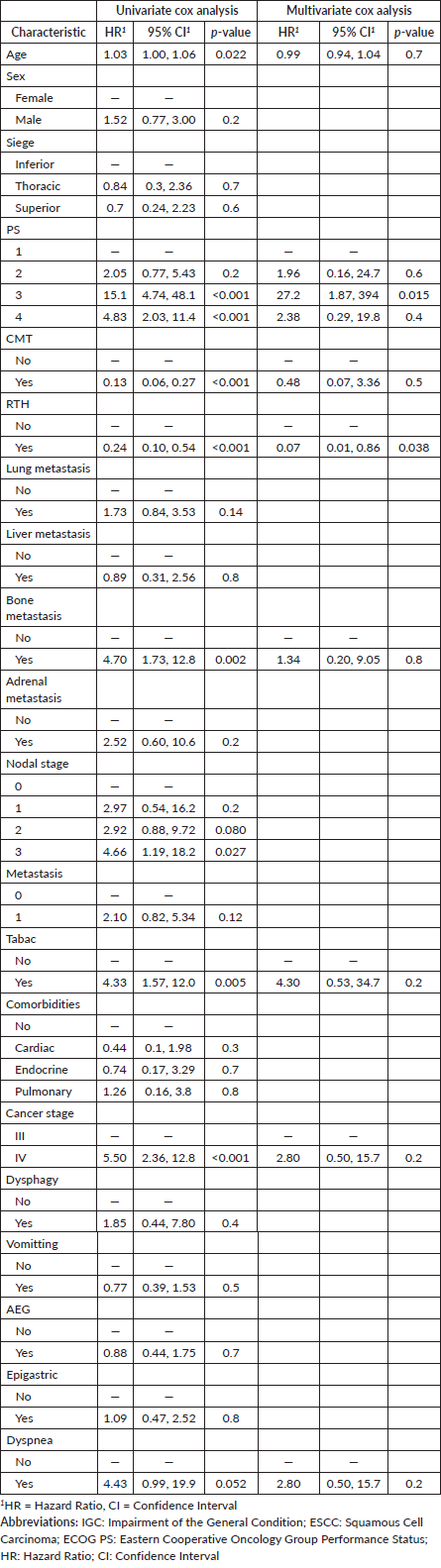

Figure 1. OS in patients diagnosed with ESCC as the primary malignancy stratified by stage at diagnosis.
Discussion
ESCC is the predominant histological subtype of EC in various regions, including Morocco and Africa. Research indicates that ESCC remains the primary subtype of EC globally, especially in regions like Eastern Asia, South Central Asia and Sub-Saharan Africa [11]. Additionally, the African ESCC corridor, spanning from Ethiopia to South Africa, highlights the disproportionately high incidence and mortality rates of EC in this region, emphasising the prevalence of ESCC [12]. Therefore, both global and regional data support the emergence of ESCC as the predominant histological subtype of EC in Morocco and Africa. This subtype is marked by high incidence rates and a generally poor prognosis worldwide [13]. Concerning Africa, currently, no studies have been reported to compare and analyse the morbidity and prognosis between the Moroccan population and other African, European, American and Asian regions [14].
Delays in diagnosis remain a significant barrier to improving outcomes for patients with ESCC in Morocco. The average diagnostic interval in Morocco surpasses international recommendations, with patients waiting approximately 52 days from symptom onset to diagnosis [14]. This delay is driven by several interrelated factors, including socioeconomic challenges, cultural and geographical barriers and systemic healthcare limitations. Socioeconomic challenges play a critical role in impeding timely diagnosis and treatment. Financial constraints and limited access to healthcare facilities disproportionately affect patients from lower-income groups, particularly those covered by the Régime d’Assistance Médicale (RAMED) system RAMED system, which is Morocco’s medical assistance program designed to provide healthcare access to economically disadvantaged populations. Economic barriers significantly hinder patients’ ability to seek and receive adequate oncology care [15]. Reliance on traditional, complementary and integrative medicine can lead to critical delays, as patients may prioritise these remedies over seeking immediate medical care [16].
In our study, the independent prognostic factors associated with survival in ESCC include the effects of RT and PS. RT has emerged as a crucial protective factor in reducing mortality risk in patients with ESCC. Studies indicate that both postoperative radiotherapy (PORT) and definitive chemoradiotherapy (dCRT) significantly enhance survival outcomes, particularly when combined with chemotherapy. PORT has been shown to improve local-regional recurrence-free survival and OS in patients undergoing surgical resection [17]. dCRT, particularly with advanced techniques like intensity-modulated radiotherapy, has demonstrated improved 5-year OS rates of 30.9% in non-surgically resectable cases [18]. Neoadjuvant chemoradiotherapy has been associated with improved surgical outcomes and survival compared to surgery alone, highlighting its role in managing locally advanced ESCC [19]. These findings suggest a more aggressive treatment approach in patients with locally advanced disease (stage III), where RT, often combined with chemotherapy, plays a central role. Palliative RT offers significant survival benefits for patients with EC, particularly in managing dysphagia, a common symptom affecting quality of life. Studies indicate that external beam radiotherapy can alleviate dysphagia in approximately 82.45% of patients, with a notable decrease in dysphagia scores post-treatment [20]. Furthermore, palliative RT has been associated with improved OS and cancer-specific survival in metastatic cases, with median OS extending significantly for those receiving radiation compared to non-receivers [21, 22]. While RT significantly enhances survival rates in ESCC, concerns regarding treatment-related toxicities persist, necessitating careful patient selection and treatment planning to optimise outcomes [17].
Research indicates that PS significantly predicts survival outcomes, influencing treatment decisions and patient stratification. PS is a critical measure of a patient’s overall health, and scales like ECOG and Karnofsky are commonly used. A study highlighted that PS significantly impacts survival, with better PS correlating with improved outcomes in various cancers, including ESCC [23]. Specifically, in patients receiving palliative treatment, a better PS was associated with 2.56 times greater odds of survival [24]. In our study, patients with PS III and IV exhibited significantly worse prognoses due to their inability to tolerate standard therapies. All patients with PS III or IV received only palliative supportive care, underscoring a critical gap in addressing the needs of this subgroup.
These findings emphasise the urgent need to develop tailored interventions, such as dose-reduced or symptom-targeted therapies, to better meet the unique needs of these patients [25, 26]. Early integration of supportive and palliative care may also improve quality of life and optimise outcomes [27]. Exploring these approaches in the Moroccan context could help address this unmet need. Increased awareness of EC risk factors has been associated with shorter intervals between symptom onset and diagnosis. This underscores the importance of community-based education programs to inform individuals about early symptoms and the need for prompt medical consultation [28, 29]. Addressing systemic barriers such as financial constraints, limited health literacy and inadequate healthcare infrastructure is crucial for reducing diagnostic delays [30, 31] Cost-effective screening methods tailored to local contexts could facilitate earlier detection, while international collaborations may help develop effective health policies and enhance cancer care capacity in low- and middle-income countries (LMICs) [31, 32]. Efforts in other LMICs, such as Kenya’s mobile diagnostic units, demonstrate the potential for community-level solutions to improve early detection [33]. Addressing these priorities is essential for improving survival outcomes and quality of life for ESCC patients in Morocco. Future research should focus on multicenter studies to validate these findings and assess the long-term impact of proposed interventions.
In our study, chemotherapy did not significantly improve survival, which may be attributed to several factors. Advanced-stage disease and poor PS significantly hinder patients’ ability to tolerate standard chemotherapy regimens, as evidenced by studies indicating that treatment delays and toxicity can diminish efficacy and OS in advanced cancer patients [34, 35]. Additionally, the concept of ‘time toxicity’ highlights the burden of healthcare interactions, which can detract from quality of life and potentially lead to treatment interruptions [36]. Furthermore, patients often prioritise quality of life over progression-free survival when faced with treatments that do not offer OS benefits, indicating a complex decision-making landscape [37].
Study limitations
The main limitations of this study include the potential for recall bias due to its retrospective design, as well as missing data for certain parameters, such as clinical T category and tobacco use. Additionally, the study was conducted at a single institution, which may not fully represent the clinical, demographic and socioeconomic diversity of the broader Moroccan population. The relatively small sample size of 78 patients further limits the generalisability of the findings, highlighting the need for larger, multicenter studies to confirm these results. The imbalance in PS scores may have introduced bias, as patients with better PS are more likely to tolerate aggressive treatments, potentially inflating survival outcomes. The high proportion of censored patients, especially in stage III, reduced the number at risk at later time points but did not affect the accuracy of the median survival, calculated using observed events with the Kaplan–Meier method. Furthermore, key risk factors, including alcohol consumption, family history, dietary habits and hot beverage consumption, were not addressed in this study due to the limitations of retrospective data and the need for prospective data collection.
Conclusion
This retrospective study underscores the poor survival outcomes and identifies key prognostic factors influencing survival in ESCC patients treated at the Mohammed VI University Hospital in the Marrakesh-Safi region of Morocco. PS is a strong independent prognostic factor for survival in ESCC, with poorer PS associated with significantly higher mortality. Early detection of EC is crucial for improving survival chances, emphasising the importance of improving access to timely diagnostic and treatment services, investing in healthcare infrastructure and enhancing oncologist training.
List of abbreviations
AJCC, American Joint Committee on Cancer; dCRT, definitive Chemo-RadioTherapy; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ESCC, Squamous Cell Carcinoma; LMICs, Low- and Middle-Income Countries; OS, Overall survival; PORT, Postoperative radiotherapy; RAMED, Régime d’Assistance Médicale (Moroccan medical assistance system); RT, Palliative radiotherapy..
Acknowledgments
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Funding
None.
Ethics approval and consent to participate
The Marrakech Faculty of Medicine and Pharmacy’s Ethical Review Committee waived its approval for the study. It was not necessary to obtain the patient’s informed permission. Before analysis, patient records were anonymised to ensure confidentiality. All methods were performed according to the relevant guidelines and regulations. The need for written informed consent was waived by The Marrakech University Hospital Ethics Committee due to the retrospective nature of the study.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analysfed during the current study are available from the corresponding author on reasonable request.
Author contributions
Conception and design: Anass Baladi, Hassan Abdelilah Tafenzi, Rhizlane Belbaraka.
Statistical analysis: Anass Baladi, Hassan Abdelilah Tafenzi.
Data interpretation: All authors.
Financial support: Anass Baladi, Hassan Abdelilah Tafenzi, Rhizlane Belbaraka.
Administrative support: Bioscience and Health Laboratory, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco & Medical Oncology Department, Mohammed VI University Hospital, Marrakech, Morocco.
Provision of study materials or patients: Anass Baladi, Hassan Abdelilah Tafenzi, Rhizlane Belbaraka.
Drafting: Anass Baladi, Hassan Abdelilah Tafenzi.
Review, revise and approve the manuscript: All authors.
Author information
Anass Baladi (anassbaladi2020@gmail.com), Medical Oncology Resident
Hassan Abdelilah Tafenzi (hassanabdelilah.tafenzi@gmail.com), PhD student
Fatim-Zahra Megzar (megzar.fatim.zahra@gmail.com), Medical Oncology Resident
Ibrahima Kalil Cisse (ibkalilcisse@gmail.com), Medical Oncology Doctor
Othmane Zouiten (drzouitenothmane@gmail.com), Professor of Medical Oncology
Leila Afani (afanileila@gmail.com), Professor of Medical Oncology
Ismail Essaadi (ismail_onco@yahoo.fr), Professor and Head of Medical Oncology department, Avicenna Military Hospital of Marrakech, Morocco
Mohammed El Fadli (elfadli.mohamed2000@gmail.com), Professor of Medical Oncology
Rhizlane Belbaraka (belbaraka.r@gmail.com), Professor and Head of Medical Oncology department, Mohammed VI University Hospital of Marrakech, Morocco.
References
1. Mazidimoradi A, Banakar N, and Khani Y, et al (2023) Current status and temporal trend in incidence, death, and burden of esophageal cancer from 1990−2019 Thorac Cancer 14(24) 2408–2458 https://doi.org/10.1111/1759-7714.15028 PMID: 37443420 PMCID: 10447176
2. Liu C, Ma Y, and Qin Q, et al (2023) Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040 Thorac Cancer 14(1) 3–11 https://doi.org/10.1111/1759-7714.14745 PMCID: 9807450
3. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
4. Simba H, Kuivaniemi H, and Abnet CC, et al (2023) Environmental and life-style risk factors for esophageal squamous cell carcinoma in Africa: a systematic review and meta-analysis BMC Public Health [Internet] 23(1) 1782 [https://www.researchsquare.com/article/rs-2946864/v1] Date accessed: 09/06/24 https://doi.org/10.1186/s12889-023-16629-0 PMID: 37710248 PMCID: 10500769
5. Rajput N, Gholap D, and Mhatre S, et al (2022) Epidemiological review: esophagus squamous cell carcinoma in India Indian J Med Paediatr Oncol 43(05) 393–403 https://doi.org/10.1055/s-0042-1755445
6. Wang L, Pang W, and Zhou K, et al (2022) Risk factors for esophageal squamous cell carcinoma in patients with head and neck squamous cell carcinoma J Oncol 2022 1–6
7. Rock CL, Thomson C, and Gansler T, et al (2020) American Cancer Society guideline for diet and physical activity for cancer prevention CA Cancer J Clin 70(4) 245–271 https://doi.org/10.3322/caac.21591 PMID: 32515498
8. Guo J, Zhang S, and Li H, et al (2021) Lung metastases in newly diagnosed esophageal cancer: a population-based study Front Oncol [Internet] 11 603953 Date accessed: 08/05/23 https://doi.org/10.3389/fonc.2021.603953 PMID: 33718154 PMCID: 7947855
9. Visaggi P, Barberio B, and Ghisa M, et al (2021) Modern diagnosis of early esophageal cancer: from blood biomarkers to advanced endoscopy and artificial intelligence Cancers 13(13) 3162 https://doi.org/10.3390/cancers13133162 PMID: 34202763 PMCID: 8268190
10. Meves V, Behrens A, and Pohl J (2015) Diagnostics and early diagnosis of esophageal cancer Viszeralmedizin 31(5) 315–318
11. Huang J, Lok V, and Zhang L, et al (2022) IDDF2022-ABS-0168 global incidence of oesophageal cancer by histological subtypes in 2020: a systematic analysis of registries Gut 71 A152.1
12. Sheikh M, Roshandel G, and McCormack V, et al (2023) Current status and future prospects for esophageal cancer Cancers 15(3) 765 https://doi.org/10.3390/cancers15030765 PMID: 36765722 PMCID: 9913274
13. Zhang Y and Chen Y (2022) Stratification from heterogeneity of the cell-death signal enables prognosis prediction and immune microenvironment characterization in esophageal squamous cell carcinoma Front Cell Dev Biol 10 855404 https://doi.org/10.3389/fcell.2022.855404 PMID: 35493093 PMCID: 9040162
14. Sauvaget C, Boutayeb S, and Bendahhou K, et al (2023) The journey of cancer patients and the quest to equity: findings from Morocco Public Health 223 33–41 https://doi.org/10.1016/j.puhe.2023.07.015 PMID: 37597462 PMCID: 10547108
15. Chadia Z, Hassane A, and Ahmed O (2023) The influence of socio-economic factors on access to oncology care among the poor and vulnerable in Morocco Rev Black Polit Econ 50(4) 424–445 https://doi.org/10.1177/00346446231164580
16. Kerouad J, Mesfioui A, and Ouasmani F, et al (2022) Predictors of patient delay among upper aerodigestive tract cancer patients in Morocco Ann Cancer Res Ther 30(2) 85–92 https://doi.org/10.4993/acrt.30.85
17. Han W, Chang X, and Zhang W, et al (2022) Effect of adjuvant radiation dose on survival in patients with esophageal squamous cell carcinoma Cancers 14(23) 5879 https://doi.org/10.3390/cancers14235879 PMID: 36497360 PMCID: 9736548
18. Lu N, Wang X, and Li C, et al (2020) Prognostic analysis of definitive radiotherapy for early esophageal carcinoma(T1-2N0M0): a multi-center retrospective study of Jing-Jin-ji Esophageal and Esophagogastric Cancer Radiotherapy Oncology Group Chin J Oncol [Internet] 42(2) 139–144 [https://rs.yiigle.com/cmaid/1183552] Date accessed: 16/11/24
19. Chan WWL, Lam KO, and Kwong DLW (2020) Radiotherapy for thoracic esophageal squamous cell carcinoma [Internet] Esophageal Squamous Cell Carcinoma ed AK Lam (New York: Springer US) [http://link.springer.com/10.1007/978-1-0716-0377-2_23] Date accessed: 16/11/24
20. Das A, Kalita AK, and Bhattacharyya M, et al (2023) External beam radiotherapy for dysphagia palliation in advanced esophageal cancer: a prospective study Asian Pac J Cancer Care 8(4) 715–719 https://doi.org/10.31557/apjcc.2023.8.4.715-719
21. Xu J, Lu D, and Zhang L, et al (2019) Palliative resection or radiation of primary tumor prolonged survival for metastatic esophageal cancer Cancer Med 8(17) 7253–7264 https://doi.org/10.1002/cam4.2609 PMID: 31612596 PMCID: 6885868
22. Li X, Zhang H, and Jia X, et al (2019) Survival benefit of radiotherapy in metastatic esophageal cancer: a population-based study Transl Cancer Res 8(4) 1074–1085 https://doi.org/10.21037/tcr.2019.06.15 PMID: 35116850 PMCID: 8797641
23. Gounant V, Brosseau S, and Guezour N, et al (2023) Populations particulières: patients de performance status 2 ou plus Rev Mal Respir Actual 15(2) eS172–eS184
24. Ferndale L, Ayeni OA, and Chen WC, et al (2023) Development and internal validation of the survival time risk score in patients treated for oesophageal cancer with palliative intent in South Africa South Afr J Surg Suid-Afr Tydskr Vir Chir 61(1) 66–74
25. Runge T and Baron TH (2018) Palliative treatment of esophageal cancer Esophageal Cancer [Internet] eds F Schlottmann, D Molena, and MG Patti (Cham: Springer International Publishing) pp 181–191 [http://link.springer.com/10.1007/978-3-319-91830-3_20] Date accessed: 01/01/25 https://doi.org/10.1007/978-3-319-91830-3_20
26. Graham L and Wikman A (2016) Toward improved survivorship: supportive care needs of esophageal cancer patients, a literature review: esophageal cancer supportive care needs Dis Esophagus 29(8) 1081–1089 https://doi.org/10.1111/dote.12424
27. Aquino JLBD and Leandro -Merhi VA (2023) Critical analysis of palliative surgical therapy for advanced thoracic esophageal cancer Seven Editora [Internet] [https://sevenpublicacoes.com.br/index.php/editora/article/view/2414] Date accessed: 01/01/25
28. Al‐Kaabi A and Siersema PD (2020) Reducing time to diagnosis in gastroesophageal cancer is key to further improve outcome United Eur Gastroenterol J 8(5) 507–508 https://doi.org/10.1177/2050640620924160
29. He Y, Li F, and Guo C, et al (2024) Dissecting the prediagnostic journey to identify opportunities for early detection of esophageal cancer: findings from a high-risk area in rural China JCO Glob Oncol 10 e2400209 https://doi.org/10.1200/GO.24.00209 PMID: 39303193
30. Khan IM, Hussain M, and Latif A, et al (2024) Factors of cancer diagnosed family and medical system for delay in diagnosis and treatment of esophageal cancer Sib J Oncol 23(3) 106–114 https://doi.org/10.21294/1814-4861-2024-23-3-106-114
31. Henke O, Qader AQ, and Malle GL, et al (2023) International cooperation to fight cancer’s late-stage presentation in low- and middle-income countries Clin Exp Metastasis 40(1) 1–3 https://doi.org/10.1007/s10585-022-10196-1 PMID: 36646888 PMCID: 9898318
32. Pubu S, Zhang JW, and Yang J (2024) Early diagnosis of esophageal cancer: how to put “early detection” into effect? World J Gastrointest Oncol 16(8) 3386–3392 https://doi.org/10.4251/wjgo.v16.i8.3386 PMID: 39171169 PMCID: 11334019
33. Dhoot R, Humphrey JM, and O’Meara P, et al (2018) Implementing a mobile diagnostic unit to increase access to imaging and laboratory services in western Kenya BMJ Glob Health 3(5) e000947 https://doi.org/10.1136/bmjgh-2018-000947 PMID: 30364326 PMCID: 6195141
34. Steventon L, Man KKC, and Nicum S, et al (2024) The impact of inter-cycle treatment delays on overall survival in patients with advanced-stage ovarian cancer Oncologist 29(11) e1532–e1539 https://doi.org/10.1093/oncolo/oyae201 PMID: 39245440 PMCID: 11546639
35. Da Silva Rocha LS, Moniz CMV, and Silva MPME, et al (2023) Effects of palliative chemotherapy in unresectable or metastatic colorectal cancer patients with poor performance status Clin Colorectal Cancer 22(3) 291–297 https://doi.org/10.1016/j.clcc.2023.05.001 PMID: 37336705
36. Duffens A, Zhu S, and Shirazi A, et al (2024) Time toxicity faced by patients with advanced cancer across primary cancer sites JCO Oncol Pract 20(10_suppl) 268 https://doi.org/10.1200/OP.2024.20.10_suppl.268
37. Brundage MD, Booth CM, and Eisenhauer EA, et al (2023) Patients’ attitudes and preferences toward delayed disease progression in the absence of improved survival JNCI J Natl Cancer Inst 115(12) 1526–1534 https://doi.org/10.1093/jnci/djad138 PMID: 37458509 PMCID: 10699849






