Subcutaneous versus intravenous administration of Trastuzumab: a minimization cost analysis with real world data from a reference cancer centre in Peru
Iris Otoya1,a, Natalia Valdiviezo1,b, Katia Roque1,2,c, Zaida Morante1,d, Tatiana Vidaurre1,e, Silvia P. Neciosup1,f, Mónica J. Calderón1,3,g and Henry L. Gomez1,3,4,h
1Departamento de Medicina Oncológica, Instituto Nacional de Enfermedades Neoplásicas, Lima 15038, Perú
2Post Graduation Programme in Medicine, UNINOVE, Sao Paulo 03155-000, Brasil
3Institute of Investigations in Biomedical Sciences (INICIB), Universidad Ricardo Palma, Lima 15039, Perú
4Oncosalud, Auna Ideas, Lima 15036, Perú
ahttps://orcid.org/0000-0001-6185-9853
bhttps://orcid.org/0000-0002-4300-5958
chttps://orcid.org/0000-0003-3267-7107
dhttps://orcid.org/0000-0003-2952-6068
ehttps://orcid.org/0000-0003-1995-4560
fhttps://orcid.org/0000-0002-2657-9853
ghttps://orcid.org/0000-0003-4935-7927
hhttps://orcid.org/0000-0003-2660-1843
Abstract
Breast cancer (BC) is a global concern, with Peru experiencing a high incidence and mortality. Trastuzumab, a crucial treatment for human epidermal growth factor receptor 2-positive BC, is administered intravenously or subcutaneously (SC). This study evaluates the costs associated with both methods at Peru's Instituto Nacional de Enfermedades Neoplásicas. Real data indicate that SC administration reduces treatment costs by approximately S/15,049.09. Cross-continental comparisons highlight a global trend favouring SC administration for efficiency and cost-effectiveness. The analysis provides insights for informed decision-making in resource-constrained healthcare settings like Peru, emphasising the need to consider local contexts in optimising oncology care.
Keywords: breast cancer, HER2 positive, Trastuzumab, subcutaneous, intravenous, cost, Peru
Correspondence to: Iris Otoya
Email: iris.otoyaf@gmail.com
Published: 31/05/2024
Received: 18/01/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
According to GLOBOCAN 2020, breast cancer (BC) diagnosis has been increasing worldwide. It ranks as the fifth leading cause of overall cancer death, but for women, BC has the highest incidence and mortality rate, with a frequency of 24.5% and 15.5%, respectively [1]. In most Latin American countries, this pattern repeats [2]. Although in Peru, stomach cancer has the highest mortality in women, BC still prevails as the most diagnosed with an incidence of 18.5% in the whole country and resulting in up to 1,825 deaths in 2020 [1]. Moreover, in Metropolitan Lima alone new cases from 2013 to 2015 accounted for 18.2% of all malignant neoplasms and 12.9% of female deaths [1, 3].
For this type of cancer, its standard practice consists of evaluating the human epidermal growth factor receptor 2 (HER2) protein’s status. HER2 is found expressed in the membrane of some cancer cells and is involved in promoting cell growth and survival; therefore, it is crucial in determining the most effective therapeutic strategy [4]. HER2 overexpression is present in approximately 15%–20% of malignant breast neoplasms and is characterised by its aggressiveness with a high recurrence rate and fairly short disease-free survival (DFS) time after adjuvant treatment [4, 5].
Trastuzumab is a humanised monoclonal antibody that, by homodimerisation, inhibits the HER2 signaling pathway that promotes growth [6]. This drug has been approved by the FDA since 1998 for the treatment of HER2-positive metastatic BC as monotherapy or in combination with chemotherapy [6, 7]. The synergistic effect of Trastuzumab with that of chemotherapy, i.e., taxanes paclitaxel and docetaxel, reduces the risk of recurrence and mortality by half, in contrast to a regimen composed by chemotherapy alone; resulting in a 10% of absolute improvement in long-term DFS and a 9% increase in 10-year overall survival [8]. Trastuzumab is also approved in patients with node-positive disease and in N0 patients with tumours >1 cm, due to the relatively high risk of relapse; in addition, it is effective for both early stage and metastatic BC [8, 9].
Trastuzumab is administered via intravenous (IV) infusion every 3 weeks with a dosage calculated according to the patient's weight [10] or employing a subcutaneous (SC) fixed-dosage formulation administered by a single-use injection device [11]. Different studies have shown that subcutaneous Trastuzumab (SC-TZM) can offer many advantages compared to intravenous Trastuzumab (IV-TZM) and that it is effective in the treatment of patients with early stage HER2-positive BC, according to the primary endpoints of pathologic complete response (absence of invasive neoplastic cells in the breast, remaining ductal carcinoma in situ) [10, 12, 13]. Likewise, there is no significant difference between the two formulations with respect to adverse effects and event-free survival rates up to 3 years after completion of treatment [12, 13].
This study’s purpose is to evaluate the costs related to the administration of intravenous and SC-TZM at a public healthcare centre in Peru. To accomplish this, we considered the time invested by our staff in preparing and administering the drug. Regarding costs, we calculated the cost of Trastuzumab, materials for drug preparation, supplies needed for administration, infusion chair use and personnel involved in the process.
Methods
The study was done in the Instituto Nacional de Enfermedades Neoplásicas (INEN), Lima–Peru; a national reference centre for the care of oncology patients.
We created a simple spreadsheet model using Microsoft Excel 2000 (Microsoft Corporation, USA) to evaluate the application costs per dosage of Trastuzumab (SC and IV presentation). All the costs used in our calculations were taken from the price list of the Department of Pharmacy of the INEN [14] based on the 2019 rate and expressed in the national currency, soles (S/.). INEN uses standardised prices for the only IV and SC trastuzumab presentations available at the said institution.
The study is focused on the healthcare payer perspective, since the costs are intended to be paid by the Seguro Integral de Salud (SIS), a Peruvian health insurance aimed at residents who do not have any other insurance. The findings will be relevant for decision-making entities that are responsible for allocating resources and funding healthcare interventions.
The time horizon for this study refers to the period of treatment (1 year) with each type of Trastuzumab administration, from the first dose to the last one (18 cycles total). The costs of treating adverse events were not included in the analysis.
To properly evaluate the cost and time of each administration, we divided it into the next elements.
The drug
The nomenclature of SC-TZM used is 120 mg/mL/5 mL in vial form. The nomenclature of IV-TZM used is 21 mg/mL/20 mL, this specific drug was a biosimilar used in vial form. A single vial of Trastuzumab has a different cost depending on the type of administration (SC-TZM: S/. 4,212, IV-TZM: S/. 4,348) [14].
The treatment protocol for SC presentation was 18 fixed doses of 600 mg with a 3-week interval between doses. SC-TZM required a single vial for each administration. For IV-TZM presentation, it was an initial dosage of 8 mg/kg of body weight followed by 17 maintenance dosages of 6 mg/kg of body weight with a 3-week interval between doses. Calculations were made considering a mean of 27.54 BMI from a previous study with patients from INEN [15] We used two vials for the first dosage and one vial for the remaining 17 doses.
All treatments were administered according to the INEN's Care and Application of Specialised Medical Oncology Treatment Protocols.
Materials for drug preparation
Cost of any material involved in the preparation of SC-TZM and IV-TZM. Calculations were made for a single dose (1 cycle) and total treatment (18 cycles).
Supplies for drug administration
Cost of any supply used in the administration of SC-TZM and IV-TZM. Calculations were made for a single dose and total treatment.
Infusion chair use
The cost of Specialised Medical Oncology Treatment Application on an outpatient by SC-TZM and IV-TZM are obtained from the Nursing and Chemotherapy Procedures tariffs from INEN. Calculations were made for a single dose and total treatment.
Personnel involved in the process
The personnel involved consisted of a pharmaceutical chemist for drug preparation and a licensed nurse specialist in Oncology for drug administration. There is a doctor scheduled for supervision in each treatment room but since they do not have a specific time designed for each patient and the monitoring is made to the whole room at once, time and cost of patient supervision were not considered in this analysis.
Drug preparation
Both drugs were stored at 2°C–8°C, following FDA recommendations and previous studies [11, 16, 17]. Since there was no difference in storage, this was not included in analysis.
Average preparation time did not include the time for transferring supplies from the warehouse to the pharmacy, the pre-labeling of the input material and the time for dressing change of the operators; only the time it took for the pharmaceutical chemist to reconstitute the IV-TZM presentation. Preparation cost was calculated using the salary per minute of a pharmaceutical chemist
Drug administration
Administration time is defined as the time from the patient sitting in the infusion chair to the moment in which they get out of the chair at the end of each treatment cycle. Administration cost was calculated using the salary per minute of a licensed nurse specialist in Oncology.
Statistical analysis
We conducted a descriptive analysis in RStudio. Packages such as ‘gtsummary’ and ‘tidyverse’ were installed to obtain tables. No other analysis were made since at the time of analysis when SC-TZM was available, there was only one provider for both SC and IV-TZM at INEN. Therefore, there was a single, fixed cost for each form of drug administration at INEN’s Department of Pharmacy.
Ethical considerations
The study was approved by the Ethics Review Board of the INEN (22-0278) and conducted in compliance with all relevant ethical guidelines.
Results
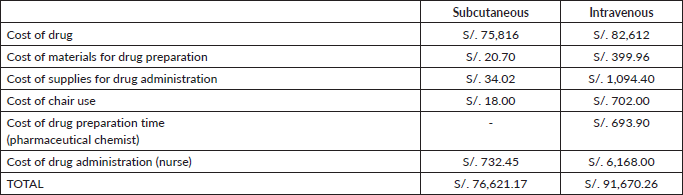
Considering the prices of the Department of Pharmacy of the INEN [14] and that IV presentation depends on body weight, as mentioned in Methods, we used the cost of two vials for the first dose of IV-TZM, S/. 8,696, and one vial for each subsequent administration, S/4,348. Given the 18 cycles of complete treatment, we have a total cost of IV-TZM being S/.73,916. On the other hand, SC-TZM costs S/.4,212 each vial and offers a fixed dosage of 600 mg, freeing the protocol from variations associated with weight. This characteristic offers greater predictability in medical expenses, avoiding the possible economic fluctuations that IV-TZM would present. SC-TZM gives a total of S/.75,816 while IV-TZM amounts to S/.82,612 (Table 1). There is a saving of S/.6,796 in favour of SC-TZM.
If we evaluate the cost assigned to materials for drug preparation for each method, we can see a substantial saving in consumables in the SC presentation, S/. 1.15, in comparison to S/.22.22 for IV-TZM in a single dose. The main difference is the price of the pump infusion line without volutrol (Table 2). The total cost for this category regarding SC-TZM is S/.20.70, meanwhile in IV-TZM is S/.399.96 (Table 3). The saving equals S/.379.26 in favour of SC-TZM.
Likewise, the cost of supplies needed for drug administration for a single-dose application is 32 times more expensive for IV-TZM than SC-TZM (S/.60.8 versus S/.1.89, respectively, Table 4), and considering the 18 cycles, this translates to a saving of S/ 1,060.38 (S/.1,094.40 versus S/.34.02, respectively, Table 5). The biggest difference in cost is due to the Dehp-Free pump infusion line for cytostatics, bifurcated extension of two needle-less valves, and sodium chloride 900 mg/100 mL (Table 4).
The cost of a Specialised Medical Oncology Treatment Application on an outpatient by SC-TZM is S/. 1.00 and by IV-TZM is S/. 39.00. In general, the cost related to the use of an armchair for SC presentation represents a saving of approximately 97% (S/.18 versus S/.702) in 18 treatment cycles compared to IV administration (Table 6).
The cost of Pharmacy Staff time, from the Central Mixing Area, and Nurse Time associated with treatment preparation was estimated by dividing the average monthly salary by the 150 hours scheduled in 1 month to calculate the hourly cost, giving a salary of S/. 23.33 and S/. 2.57 per hour and minute, respectively (Table 7).
Each vial for IV-TZM application is reconstituted with 7.2 mL of sterile water per injectable preparation for a single dose containing 21 mg/mL of Trastuzumab. One should not shake the product as excessive foaming during reconstitution could cause problems with the amount of Trastuzumab that can be extracted from the vial. If this occurs, the vial must be left resting for approximately 5 minutes [18]. Considering those recommendations, we have a total preparation time of 15 minutes for each IV-TZM application. Meanwhile, SC-TZM does not need any preparation time. Then, the time for SC-TZM is 270 minutes less in total (Table 8). The Preparation cost is S/.38.55 per cycle for IV-TZM, giving a saving of S/. 639.90 using the calculation of the salary per minute of a pharmaceutical chemist (Table 9).
The drug administration time is defined as the time from the patient sitting in the infusion chair to the moment in which they get out of the chair at the end of each treatment cycle. For IV presentation, the starting dosage should be administered as an IV infusion over 90 minutes. Patients are observed for 6 hours since the start of the first infusion and for 2 hours since the start of subsequent infusions for symptoms such as fever and chills or other infusion-related symptoms. If the starting dosage is well tolerated, subsequent doses may be administered as a 30-minute infusion [18]. For SC presentation, the recommended dosage is 600 mg administered by SC injection (under the skin) over 2–5 minutes. Monitoring is needed in case of adverse effects during administration and for at least 30 minutes after the first administration and for 15 minutes after subsequent administrations [18].
Then, SC-TZM drug administration time lasts 35 hours and 15 minutes less than IV-TZM (4 hours and 45 minutes versus 40 hours, Table 10). The cost associated with these times, using the calculation of the salary per minute of a licensed nurse, is S/. 732.45 for SC-TZM and S/.6 168.0 for IV-TZM (Table 11), giving a saving of S/. 5,435.55.
In summary, we spent S/. 76,621.17 in the whole treatment with SC-TZM and S/. 91,670.26 in IV-TZM (Table 12), with a total difference of S/. 15,049.09 which equals a saving of 16.42%.
Discussion
Approved initially by all major regulatory agencies for the treatment of HER2+ metastatic BC, trastuzumab was expanded in 2006 for the treatment of early-stage HER2-positive BC [6, 9]. Adding trastuzumab to chemotherapy has improved the prognosis of this population. The evidence shows a significant reduction in recurrence (RR 0.66, 95% CI 0.62–0.71; p < 0.0001) and death (RR 0.67, 95% CI 0.61–0.73; p < 0.0001) compared to chemotherapy alone [9, 19, 20]. Furthermore, the demonstrated clinical effectiveness and safety of the medication reinforces its significance in managing early-stage BC [21, 22].
Table 1. Cost of drug by presentation (expressed in local currency: Soles).
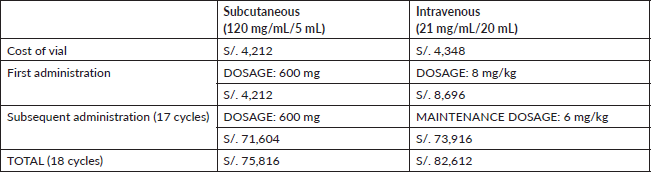
Table 2. Cost of materials for drug preparation for a single dose (expressed in local currency: Soles).
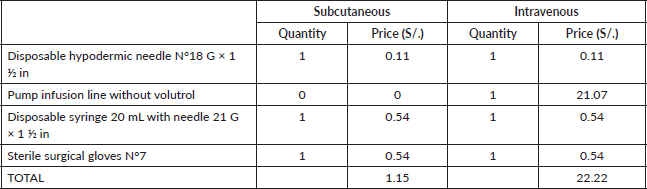
Table 3. Total cost of materials for drug preparation (expressed in local currency: Soles).

Table 4. Cost of supplies for drug administration for a single-dose (expressed in local currency: Soles).
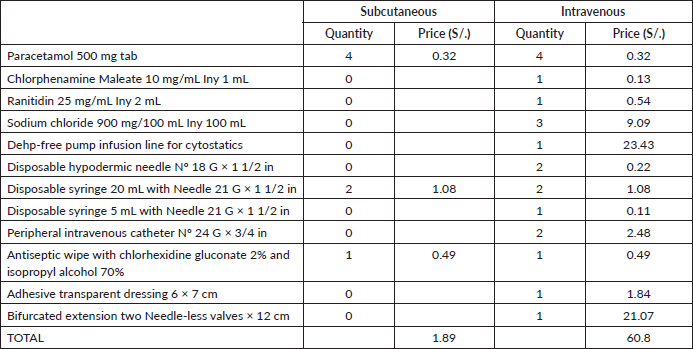
Table 5. Total cost of supplies for drug administration (expressed in local currency: Soles).

Table 6. Cost of chair use (expressed in local currency: Soles).

Table 7. Cost of personnel involved (expressed in national currency: Soles).
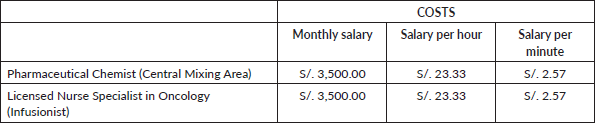
Table 8. Total time of drug preparation.
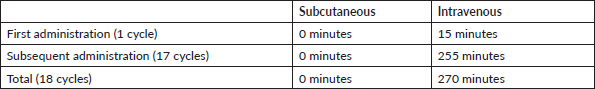
Table 9. Total cost of drug preparation time (expressed in national currency: Soles).

Table 10. Total time of drug administration.

Table 11. Total cost of drug administration.
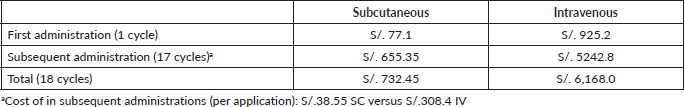
Table 12. Final cost of treatment by presentation.
Biosimilar drugs, with comparable efficacy and safety to original biologics but lower production costs, are being increasingly integrated into clinical practice [23]. In HER2+ early BC, trastuzumab biosimilars have shown similar outcomes to the original product [24, 21]. Despite their potential to improve cancer care affordability, biosimilar uptake in Latin America, including Peru, has been slow due to various barriers such as regulations, legislature and market opportunities [25]. Currently, Peru has registered only two trastuzumab biosimilars of intravenous presentation since 2019 [14, 26]. Even though biosimilars represent lower spending costs, SC-TZM may offer potential time and cost-associated advantages, that are particularly important in resource-limited settings such as our country, where SC-TZM was only available for a short time during 2019.
Effective management of limited resources is critical to ensuring treatment access in public facilities, highlighting the critical role of economic evaluation in trastuzumab uptake and access in INEN, Peru. The limited public funding provided by SIS requires well-informed managerial decisions based on the costs associated with different ways of administering drugs [27, 28]. This evaluation aims to inform public management decisions and to add to the existing literature on the costs of oncology treatment, which can influence decisions on allocating resources in the health care system.
During our study, we examined real data to compare the expenses of administering 18 cycles of intravenous versus SC-TZM to patients with adjuvant HER2-positive BC. Our findings indicate that SC application reduces total treatment costs by approximately S/15,049.09, approximately 4,071.72 USD. A previous study in Peru, which used a simulation for cost analysis, estimated a direct cost reduction of S/.246.812,20 for the treatment of 100 patients with SC-TZM or S/.2,468.11 per patient [28]. It should be recognised that simulations might not capture all the variability and complexity of the real system, which may limit the extrapolation of results. However, the actual data reflect current conditions. The convergence of the results suggests that there is a consistent trend toward the economic advantages of the SC route of administration. In addition, it was observed that patient weight has a significant impact on the dosing of IV-TZM, as 80% of the patients weighed over 55 kg and thus required two doses of IV-TZM. This finding is of great importance in the Peruvian context where, according to the report ‘Perú: Enfermedades no transmisibles y transmisibles – 2021,’ 36.9% of the adult population is overweight and the average weight of women is 57 kg [29, 30]. The weight-based dosing of IV-TZM, which means that IV-modality may be comparatively more expensive in Peru, increases the cost.
The administration time of an oncology treatment is significantly influenced by the choice between subcutaneous and IV-TZM. Administration of SC-TZM reduces the administration time by 330 minutes because the drug is available in a ready-to-use formulation, eliminating the drug preparation process and subsequently reducing the use of clinical resources such as chemotherapy chairs and the time of healthcare personnel. Similarly, a previous Peruvian study found that, although the SC-TZM resulted in a longer post-injection monitoring time, the time spent by nursing staff was reduced due to the lack of infusion equipment, indicating a benefit for the SC-TZM. This pooled analysis highlights the significance of including direct administration times as well as operational and monitoring aspects when assessing the benefits of various modalities for administering trastuzumab [28].
Similarly, Lopez-Vivanco et al [31], calculated the healthcare professional’s time (HCP) as the mean sum of task times for all IV and SC administrations, the mean patient infusion chair time and treatment room time. The transition from IV to SC trastuzumab reduced active HCP time by half (27.2 minutes versus 13.2 minutes per cycle) due to the lack of IV catheter use, line flushing and drug reconstitution. A 78%–85% reduction in chair time and a 59%–81% reduction in patient’s treatment room time with SC-TZM resulted in 24 hours of free time for the 18 cycles. The respective saving was €979.60 for one complete treatment cycle [31].
Based on McCloskey et al [32] systematic review, we conducted a cross-continental comparison to determine similarities and differences in the trends of administering SC and IV Trastuzumab. This comparison aims to understand how global discoveries are reflected in different regions and how these might be contextualised in the Peruvian setting, thus providing a more comprehensive perspective [32].
In Europe, studies have shown SC administration to be more efficient and less expensive. For example, in Germany, trastuzumab SC administration has a significantly shorter total time of 5.4 minutes compared to 30 minutes for the IV formulation [33]. In Italy, when evaluating three different scenarios: IV-TZM, SC-TZM and IV-TZM followed by SC-TZM, they found that SC-TZM saved the most time. The reduction was 71.7% and 89.3% in preparation time and chair time use, respectively, compared to IV-TZM. However, total mean costs were not significant (IV-TZM: €14,233; SC-TZM: €14,272; and IV-TZM+SC-TZM: €14,535; p = 0.959), SC administration still proved to be a valuable option [34].
The results indicate a significant reduction in both time and cost, which suggests a positive bias toward implementing the SC formulation. When compared to data from Oceania, specifically New Zealand, a similar trend was observed, showing a reduction in both chair time and total nursing time. This resulted in a financial benefit of $76.94 (in New Zealand dollars) per patient per cycle [35].
A study conducted in Chile included direct and indirect medical costs associated with the preparation and administration of Trastuzumab (adjusted for body weight) and also costs due to serious adverse drug reactions and non-medical costs that occurred during the course of the 18 cycles. Results were primarily due to the number of vials per body weight [36]. Given the tendency for overweight in our patients, the use of SC-TZM instead of IV-TZM may have a significant economic impact on our public health system. Likewise, Brazil, Mexico and Panama have also shown reductions in total treatment costs and preparation and administration time with the use of SC administration [32, 37, 38]. These results confirm the operational and economic benefits of using the SC formulation, showing a comparable pattern to our results and those observed in other regions.
This comparative enhances comprehension regarding how the advantages of the subcutaneous formulation of trastuzumab are mirrored across various healthcare systems, and how these results can guide clinical and policy determinations in managing HER2-positive BC. However, there was variability in data among various research and medical entities, emphasizing the necessity of taking into account the local context, clinical protocols and evaluation methods in interpreting outcomes and devising SC therapy implementation strategies.
In addition, there is a preference for SC-TZM versus IV-TZM administration in patients with HER2-positive early BC. In 2013, 124 patients were randomised to receive SC-TZM followed by IV-TZM, and 124 to receive the reverse sequence. SC-TZM via the single-use injection device was preferred by 216 patients (p < 0.0001). Also, the reported adverse events occurred in 58% of patients during the combined SC periods and in 44% of patients during the combined IV periods; in which only 3% and 2%, respectively, were grade 3, mostly influenza; with no grade 4 or 5 events [39]. Therefore, patient preference makes an important point to consider at the moment of deciding on treatment, since not only reduces costs and improves time use, but also the patient’s disposition and quality of life.
Conclusion
The decision to deliver Trastuzumab SC or IV requires a comprehensive evaluation of time efficiency, cost, clinical need, patient preference and a framework that promotes optimal oncologic care and resource allocation. This analysis provides valuable information to inform current clinical practice and to inform future research aimed at optimising oncology care.
The results obtained within the study showed that the use of SC-TZM, compared to the IV version, generates less costs associated with an overall saving of 16.42% (18 cycles), which means less time spent in the preparation and application of the treatment, as well as a reduction in patient’s chair time. These time reductions translate into financial savings, more efficient use of resources and improved quality of care.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
This study is self-funded. The authors declare they have no financial or personal relationships with other organisations that could inappropriately influence their work.
Author contributions
All authors conceived and designed the study. All authors contributed to the article and approved the final version of the manuscript.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Torres-Román JS, Ybaseta-Medina J, and Loli-Guevara S, et al (2023) Disparities in breast cancer mortality among Latin American women: trends and predictions for 2030 BMC Public Health 23(1) 1449 https://doi.org/10.1186/s12889-023-16328-w PMID: 37507674 PMCID: 10386226
3. Payet Meza E, Sarria Bardales G, and Dunstan Yataco J, et al (2021) Registro de cáncer de Lima Metropolitana: Incidencia y mortalidad 2013–2015 [Internet] (Lima: Ministerio de Salud) [https://portal.inen.sld.pe/wp-content/uploads/2022/01/REGISTRO-DE-CANCER-DE-LIMA-METROPOLITANA-2013-2015.pdf] Date accessed: 24/06/22
4. Krishnamurti U and Silverman JF (2014) HER2 in breast cancer: a review and update Adv Anat Pathol 21(2) 100–107 https://doi.org/10.1097/PAP.0000000000000015 PMID: 24508693
5. Slamon DJ, Clark GM, and Wong SG, et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene Science (1979) 235(4785) 177–182 https://doi.org/10.1126/science.3798106 PMID: 3798106
6. Siegel J (1998) Herceptin [FDA approval letter] (Rockville: U.S. Food and Drug Administration) [Internet] [https://www.accessdata.fda.gov/drugsatfda_docs/appletter/1998/trasgen092598l.pdf] Date accessed: 21/04/24
7. Hartkopf A, Brendel M, and Wallwiener M, et al (2014) Trastuzumab administration in patients with metastatic breast cancer – experience of a Large University Breast Center Geburtshilfe Frauenheilkd 74(06) 563–568. https://doi.org/10.1055/s-0034-1368244 PMID: 24976638 PMCID: 4069214
8. Cardoso F, Kyriakides S, and Ohno S, et al (2019) Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up Ann Oncol 30(8) 1194–1220 https://doi.org/10.1093/annonc/mdz173 PMID: 31161190
9. Ali S, Hendry J, and Le D, et al (2022) Efficacy of adjuvant trastuzumab in women with HER2-positive T1a or bN0M0 breast cancer: a population-based cohort study Sci Rep 12(1) 1068 https://doi.org/10.1038/s41598-022-05209-8 PMID: 35058536 PMCID: 8776836
10. Ismael G, Hegg R, and Muehlbauer S, et al (2012) Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial Lancet Oncol 13(9) 869–878 https://doi.org/10.1016/S1470-2045(12)70329-7 PMID: 22884505
11. Genentech, Inc. Highlights of Prescribing Information: Herceptin HylectaTM (trastuzumab and hyaluronidase-oysk) Injection, for Subcutaneous Use [Internet] (U.S. Food and Drug Administration website). [https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761106s000lbl.pdf] Revised: February 2019. Date accessed: 02/11/23
12. Jackisch C, Hegg R, and Stroyakovskiy D, et al (2016) HannaH phase III randomised study: association of total pathological complete response with event-free survival in HER2-positive early breast cancer treated with neoadjuvant–adjuvant trastuzumab after 2 years of treatment-free follow-up Eur J Cancer 62 62–75 https://doi.org/10.1016/j.ejca.2016.03.087 PMID: 27208905
13. Jackisch C, Stroyakovskiy D, and Pivot X, et al (2019) Subcutaneous vs intravenous Trastuzumab for patients with ERBB2-positive early breast cancer JAMA Oncol 5(5) e190339 https://doi.org/10.1001/jamaoncol.2019.0339 PMID: 30998824 PMCID: 6512301
14. INEN (2019) Department of Pharmacy: List of prices (Lima: INEN)
15. Zavala VA, Casavilca-Zambrano S, and Navarro-Vásquez J, et al (2023) Breast cancer subtype and clinical characteristics in women from Peru Front Oncol 13 938042 https://doi.org/10.3389/fonc.2023.938042 PMCID: 10013058
16. Genentech, Inc. Highlights of Prescribing Information: Herceptin® (trastuzumab) for Injection, for Intravenous Use [Internet] (U.S. Food and Drug Administration website). [https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5345lbl.pdf] Revised: November 2018. Date accessed: 30/04/24
17. Pabari RM, Ryan B, and Ahmad W, et al (2013) Physical and structural stability of the monoclonal antibody, trastuzumab (Herceptin®), intravenous solutions Curr Pharm Biotechnol 14(2) 220–225 PMID: 23360264
18. EMA Herceptin, European Medicines Agency [Internet] (Amsterdam: EMA) [https://www.ema.europa.eu/en/medicines/human/EPAR/herceptin] Date accessed: 02/11/23
19. Bradley R, Braybrooke J, and Gray R, et al (2021) Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials Lancet Oncol 22(8) 1139–1150 https://doi.org/10.1016/S1470-2045(21)00288-6
20. Pernas S and Tolaney SM (2021) Management of early-stage human epidermal growth factor receptor 2–positive breast cancer JCO Oncol Pract 17(6) 320–330 https://doi.org/10.1200/OP.21.00020 PMID: 34111378
21. Cargnin S, Il Shin J, and Genazzani AA, et al (2020) Comparative efficacy and safety of trastuzumab biosimilars to the reference drug: a systematic review and meta-analysis of randomized clinical trials Cancer Chemother Pharmacol 86(5) 577–588 https://doi.org/10.1007/s00280-020-04156-3 PMID: 33005979
22. Kast K, Schoffer O, and Link T, et al (2017) Trastuzumab and survival of patients with metastatic breast cancer Arch Gynecol Obstet 296(2) 303–312 https://doi.org/10.1007/s00404-017-4421-x PMID: 28616827
23. Migliavacca Zucchetti B, Nicolò E, and Curigliano G (2019) Biosimilars for breast cancer Expert Opin Biol Ther 19(10) 1015–1021 https://doi.org/10.1080/14712598.2019.1638362 PMID: 31248290
24. Yang C, Khwaja R, and Tang P, et al (2022) A review of trastuzumab biosimilars in early breast cancer and real world outcomes of neoadjuvant MYL-1401O versus reference trastuzumab Curr Oncol 29(6) 4224–4234 https://doi.org/10.3390/curroncol29060337 PMID: 35735446 PMCID: 9221768
25. Teran E, Gomez H, and Hannois D, et al (2022) Streamlining breast cancer and colorectal cancer biosimilar regulations to improve treatment access in Latin America: an expert panel perspective Lancet Oncol 23(7) e348–e358 https://doi.org/10.1016/S1470-2045(22)00121-8 PMID: 35772466
26. Acción Internacional para la Salud (2021) Agilizar ingreso de biosimilares [Internet] (Lima: Acción Internacional para la Salud) [https://aisperu.org.pe/wp-content/uploads/2021/06/Nota_informativa_BIO.pdf] Date accessed: 24/04/24
27. Cañizares Fuentes WR (2018) Evolución del sistema de salud de Perú: buenas prácticas y desafíos en su construcción. Década 2005-2014 An Fac Med 78(4) 445 https://doi.org/10.15381/anales.v78i4.14269
28. Olivera Changra H and Robles Díaz JF (2022) Costos de la administración intravenosa vs. subcutánea del trastuzumab en pacientes peruanas con cáncer de mama HER2 positivo. Un análisis observacional de los costos directos e indirectos J Healthc Qual Res 37(3) 147–154 https://doi.org/10.1016/j.jhqr.2021.10.008
29. Instituto Nacional de Estadística e Informática (2021) Perú: Enfermedades no transmisibles y transmisibles (Lima: Instituto Nacional de Estadística e Informática) [https://cdn.www.gob.pe/uploads/document/file/3098590/Per%C3%BA%3A%20Enfermedades%20No%20Transmisibles%20y%20Transmisibles%2C%202021%20%28Parte%201%29.pdf?v=1652474002] Date accessed: 02/11/24
30. Instituto Nacional de Estadística e Informática (2002) Las Proyecciones de Población revelan que hay 13 millones 294 Mil Mujeres en el Perú (Lima: Instituto Nacional de Estadística e Informática) [https://www.inei.gob.pe/media/MenuRecursivo/boletines/4348.pdf] Date accessed: 17/01/24
31. Lopez-Vivanco G, Salvador J, and Diez R, et al (2017) Cost minimization analysis of treatment with intravenous or subcutaneous trastuzumab in patients with HER2-positive breast cancer in Spain Clin Transl Oncol 19(12) 1454–1461 https://doi.org/10.1007/s12094-017-1684-4 PMID: 28577152 PMCID: 5700215
32. McCloskey C, Ortega MT, and Nair S, et al (2023) A systematic review of time and resource use costs of subcutaneous versus intravenous administration of oncology biologics in a hospital setting Pharmacoecon Open 7(1) 3–36 https://doi.org/10.1007/s41669-022-00361-3
33. Hedayati E, Fracheboud L, and Srikant V, et al (2019) Economic benefits of subcutaneous trastuzumab administration: a single institutional study from Karolinska University Hospital in Sweden PLoS One 14(2) e0211783 https://doi.org/10.1371/journal.pone.0211783 PMID: 30716137 PMCID: 6361452
34. Farolfi A, Silimbani P, and Gallegati D, et al (2017) Resource utilization and cost saving analysis of subcutaneous versus intravenous trastuzumab in early breast cancer patients Oncotarget 8(46) 81343–81349 https://doi.org/10.18632/oncotarget.18527 PMID: 29113393 PMCID: 5655288
35. North RT, Harvey VJ, and Cox LC, et al (2015) Medical resource utilization for administration of trastuzumab in a New Zealand oncology outpatient setting: a time and motion study Clinicoecon Outcomes Res 7 423–430 PMID: 26251623 PMCID: 4524269
36. Rojas L, Muñiz S, and Medina L, et al (2020) Cost-minimization analysis of subcutaneous versus intravenous trastuzumab administration in Chilean patients with HER2-positive early breast cancer PLoS One 15(2) e0227961 https://doi.org/10.1371/journal.pone.0227961 PMID: 32023267 PMCID: 7001963
37. Villarreal-Garza C, De la O-Maldonado C, and Díaz-Pérez H, et al (2019) Cost and time savings of subcutaneous trastuzumab (SC-T) in a public health system in Mexico J Clini Oncol 37(15_suppl) e18387–e18387 https://doi.org/10.1200/JCO.2019.37.15_suppl.e18387
38. Martin C, Alcedo J, and Araúz E (2018) Abstract P4-12-13: comparative analysis of costs between subcutaneous formulation of trastuzumab versus intravenous formulation from the perspective of the Instituto Oncológico Nacional of Panamá from January to December 2016 Cancer Res 78(4_Supplement) P4-12-13-P4-12–3 https://doi.org/10.1158/1538-7445.SABCS17-P4-12-13
39. Pivot X, Gligorov J, and Müller V, et al (2013) Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study Lancet Oncol 14(10) 962–970 https://doi.org/10.1016/S1470-2045(13)70383-8 PMID: 23965225






