Clinical characteristics, outcomes and prognostic factors in KRAS mutant lung cancers: experience from a tertiary care cancer center in India
Vanita Noronha†, Laboni Sarkar†, Vijay Patil, Nandini Menon, Minit Shah, Akash Pawar, Oindrila Roy Chowdhury, Omshree Shetty, Anuradha Chougule, Pratik Chandrani, Rajiv Kaushal, Trupti Pai, Amit Janu, Nivedita Chakrabarty and Kumar Prabhash
Tata Memorial Hospital, Mumbai 400012, India
†The authors contributed equally to the work.
Abstract
Objectives: Kirsten rat sarcoma viral oncogene homologue (KRAS) mutations in lung cancers, long considered untargetable, have had a recent rise in interest due to promising data of agents targeting KRAS p.G12C. As Indian data are scarce, we sought to identify baseline clinical characteristics, prognostic factors and outcomes of lung cancer patients with KRAS mutations at our hospital.
Methods: Patients with KRAS mutant lung cancers treated at our institute from 2016 to 2022 were analysed.
Results: 133 patients with KRAS mutant lung cancers were identified. Median age was 57 (interquartile range 28–78) years, and 58 (43.6%) were smokers. 17 (12.7%) had brain metastases. The commonest variant was p.G12C, seen in 53 (39.8%) patients. Six (4.5%) had programmed death ligand 1 (PDL-1) expression >50% by Ventana SP263 PDL-1 assay, and 13 (9.7%) had epidermal growth factor mutation. Of 92 patients with available treatment details, the majority received intravenous chemotherapy, nine (9.8%) received tyrosine kinase inhibitors and four (4.4%) received immunotherapy (pembrolizumab). Median progression-free survival (PFS) with first-line therapy was 6 (95% confidence interval (CI) 2.8–9.2) months and median overall survival (OS) was 12 (CI 9.2–14.8) months. The incidence of brain metastases was higher in patients with G12C mutations (p = 0.025). Brain metastases (HR: 3.57, p < 0.001), Eastern Cooperative Oncology Group performance status (PS) ≥ 2 (HR: 2.13, p = 0.002) and G12C mutation (HR: 1.84, p = 0.011) were associated with inferior PFS, while brain metastases (HR: 4.6, p < 0.001), PS ≥ 2 (HR: 2.33, p = 0.001) and G12C mutation (HR: 1.93, p = 0.01) were associated with inferior OS.
Conclusion: This is the largest dataset of KRAS mutant lung cancers from India. Brain metastases were higher in patients with G12C mutations and associated with poorer PFS and OS. G12C mutation and PS ≥ 2 were also associated with inferior PFS and OS. Experience with targeted therapy for KRAS mutations remains an area of future exploration due to the unavailability of these agents in India.
Keywords: KRAS mutations, non-small cell lung cancer, real-world outcomes
Correspondence to: Kumar Prabhash
Email: kumarprabhashtmh@gmail.com
Published: 22/02/2024
Received: 02/12/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
KRAS mutations were first identified in lung cancers more than three decades ago [1–3]. There has been a recent resurgence in interest in this subset of patients with lung cancer due to the development of agents targeting KRAS G12C, with promising phase 2 data for sotorasib [4, 5] leading to accelerated approval from the United States Food and Drug Administration in patients with locally advanced and metastatic KRAS mutant non-small cell lung cancer (NSCLC) who have progressed on a first-line agent. Most patients with KRAS mutations have a history of smoking and have had historically poorer prognoses relative to those with epidermal growth factor (EGFR) mutations. There is evidence for a lack of survival benefit from adjuvant chemotherapy in resected KRAS mutant lung cancers, and resistance to erlotinib or gefitinib [6–9].
In view of the paucity of data for Indian patients, we have sought to delineate baseline clinical characteristics, treatment courses, prognosis and outcomes of lung cancer patients with KRAS mutations at our hospital, a tertiary care cancer center in India. This article is being presented in accordance with the STROBE reporting checklist.
Methods
This retrospective study involved patients with KRAS mutant lung cancers, identified from the database of patients with lung cancer from our institutional molecular tumour board clinic, wherein patients for whom next-generation sequencing (NGS) has been performed on baseline biopsy samples are discussed in a multidisciplinary meeting for therapeutic decision-making approaches at Tata Memorial Hospital. Clinical information was collected from individual patient electronic medical records including demographic data, baseline characteristics including smoking status, histopathology, radiological findings and clinical outcomes including toxicity assessment, progression, therapy at progression and survival. Patients for whom treatment records or follow-up data were unavailable and those with tumour histology other than non-small cell lung carcinoma and with EGFR and anaplastic lymphoma kinase (ALK) mutations were excluded from the survival analysis. The response was evaluated according to RECIST v 1.1. Toxicity was graded according to CTCAE, v5.0. Progression was defined as radiological progression, change of therapy or death from any cause. Computed tomography scans were done every 2–4 months or depending on patient’s symptoms. Progression-free survival (PFS) was calculated from the date of starting first-line therapy to disease progression or death. Overall survival (OS) was calculated from the date of registration to death from any cause.
Molecular testing
Patients included had undergone NGS on formalin-fixed paraffin embedded samples using the institutional standard targeted gene panel SOPHiA Solid Tumor plus Solution, which identifies 139 RNA fusions, single nucleotide variants, gene amplification events in 24 genes, indels from 42 genes and microsatellite instability (MSI) status (six unique loci) following library preparation of the extracted DNA and RNA with paired end sequencing done on the Illumina MiSeq platform and data analysis using SOPHiA DDM software. Of patients who had undergone PDL-1 testing, this was performed by the Ventana SP263 PD-L1 assay.
Statistical analysis
The statistical analysis was done using SPSS v 25. For descriptive statistics, continuous variables were reported as median with inter-quartile range (IQR) and categorical variables as frequencies and percentages. Pearson’s chi-square test was used to assess the association between two categorical variables. Two sample z tests were used to assess the difference between the proportions with the significance level set at 0.05. PFS and OS were evaluated by the Kaplan–Meier method. If the patient was lost to follow-up, the date of the last entry on their electronic medical records was taken as their last follow-up date. The Kaplan–Meier curve was plotted for the PFS and the OS over time in months [10, 11]. Log-rank test was used to compare the PFS and OS between different groups. The effect of covariates on survival was estimated using Cox proportional hazards model [12, 13].
Results
From September 2016 to July 2022, a total of 133 patients with KRAS mutant cancers were identified (Figure 1).
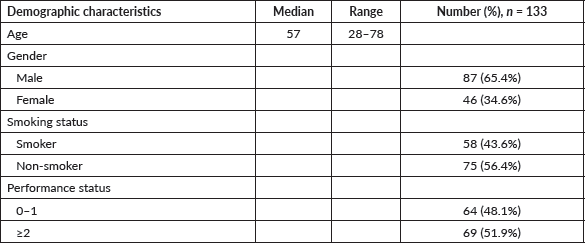
Demographic characteristics are detailed in Table 1. Median age was 57 (IQR 28–78) years. 87 (65.4%) patients were male. 58 (43.6%) had a history of smoking. Smokers had a mean of 33 (range 8–84) pack-years. Three patients had a history of intake of smokeless tobacco. 69 (51.9%) had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 or above.

Figure 1. Identification of cases. A consort diagram detailing the patients screened after keyword search, with the number of patients excluded from the survival analysis and reasons for exclusion.
Table 1. Demographic characteristics.
Clinical characteristics are detailed in Table 2. 117 (87.9%) patients had metastatic disease at baseline. The commonest site of metastases was lung, in 45 (33.8%) patients, followed by non-regional lymph nodes in 41 (30.8%) and pleural in 40 (30.1%). 17 (12.8%) patients had brain metastases. The mean tumour size was 57.5 mm (IQR 32.3–91.9).
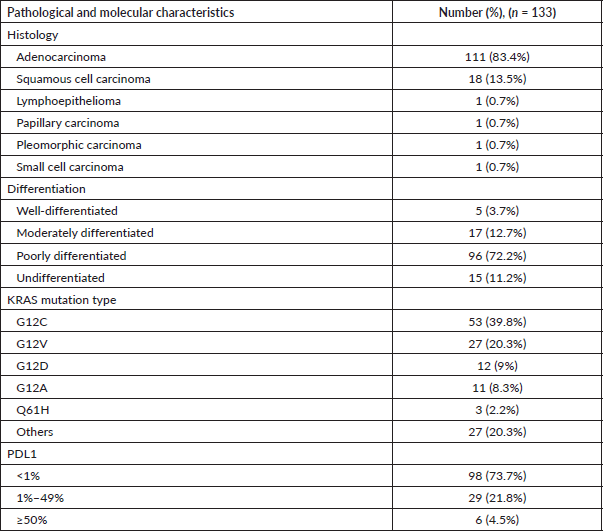
The most frequent pathology was adenocarcinoma, seen in 111 (83.4%) patients, followed by squamous cell carcinoma in 18 (13.5%). The most common type of mutation was G12C, seen in 53 (39.8%). Other mutations were found in 54 (40.6%) patients – commonest among these was the TP53 mutation, found in 16 (12%) patients. 6 (4.5%) had PDL1 expression >50% by the Ventana SP263 assay, 13 (9.7%) had EGFR mutations and 2 (1.5%) had ALK rearrangements. Molecular and pathological characteristics are listed in Table 3. Serine/threonine kinase 11 (STK11)/liver kinase B1 (LKB1) and Kelch-like ECH-associated protein 1 (KEAP1) mutations were present in 29 (21.8%) and 16 (12.0%) patients, respectively.
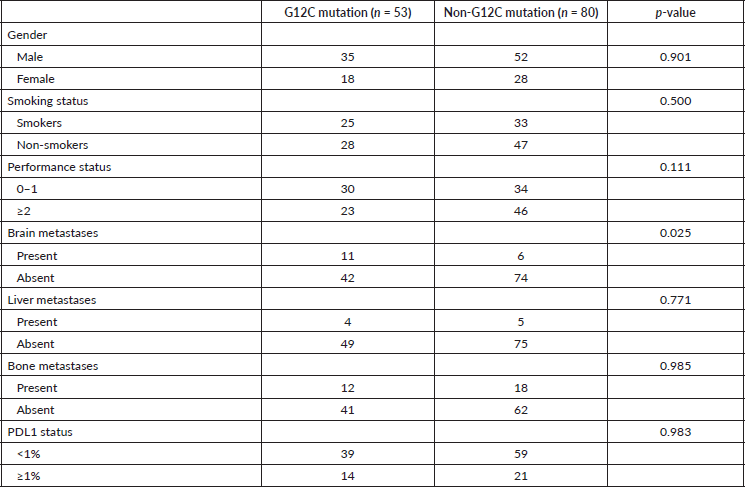
The demographic, radiological, pathological and clinical characteristics of the tumours were also compared between G12C and non-G12C subtypes using the Pearson chi-square test. 25 of 53 (47.1%) patients in the G12C group and 33 of 80 (41.3%) patients in the non-G12C group had a history of smoking (p = 0.500). 11 (20.7%) of G12C mutant cases and 6 (7.5%) of non-G12C mutant cases had brain metastases (p = 0.025) (Table 4). Among the radiological and pathological characteristics, no significant difference was observed between the subtypes.
Treatment details
Data on first-line treatment regimens were available for 92 patients, treated with non-curative intent. The commonest treatment modality was IV chemotherapy, received by 80 (86.9%) patients, most frequently used regimen being pemetrexed-carboplatin, used in 66 (71.7%) patients. This was combined with antiangiogenic targeted therapy (bevacizumab) in four patients, and immunotherapy (pembrolizumab) in four patients. Nine received first-line therapy with tyrosine kinase inhibitors. There were two patients with concomitant ALK rearrangements who received afatinib and ceritinib, and erlotinib and gefitinib were offered to four and three patients respectively on a compassionate basis. As sotorasib is unavailable in India, we were unable to offer this to our patients routinely. One patient received sotorasib for 6 months in the second-line setting after acquisition from abroad, with best response achieved being a partial response.
Table 2. Tumour characteristics.
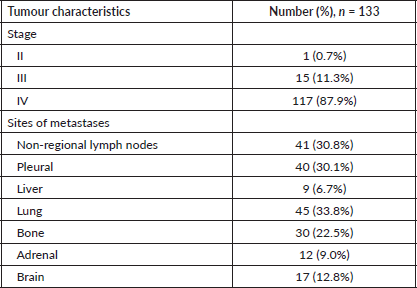
Table 3. Pathological and molecular characteristics.
Survival
The survival analysis included 76 patients who received treatment for non-curative disease with available treatment and follow-up records, after excluding patients with EGFR and ALK mutations and non-NSCLC histology. With a median follow-up of 14.2 (IQR 2–71 months), median PFS with first-line chemotherapy was 6 (95% confidence intervals (CI), 2.8–9.2) months (Figure 2a). Median OS was 12 (CI 9.2–14.8) months (Figure 2b). There were 67 deaths by the date of data-cutoff time. Patients with brain metastases (n = 12) had a median PFS of 4 (CI 1–7) months and median OS of 4.5 (CI 2–11) months, while those without (n = 64) had a median PFS of 9 (CI 6–12) months and median OS of 13 (CI 11–16) months. Patients with G12C mutations (n = 34) had a median PFS of 4.5 (CI 4–9) months, while those with non-G12C mutations had a median PFS of 10 (CI 6–15) months (Figures 3a–d and 4a–d).
By log-rank test, the presence of brain metastases (HR: 3.57, CI 1.81–7.03, p < 0.001), PS ≥ 2 (HR: 2.13, CI 1.33–3.4, p = 0.002) and G12C mutation (HR: 1.84, CI 1.15–2.96, p = 0.011) were associated with inferior PFS, while brain metastases (HR: 4.6, CI 2.29–9.21, p < 0.001), PS ≥ 2 (HR: 2.33, CI 1.41–3.85, p = 0.001) and G12C mutation (HR: 1.93, CI 1.17–3.17, p = 0.01) were associated with inferior OS (Figure 5a and b).
Table 4. Correlation between clinicopathological characteristics and type of KRAS mutation (n = 133).
Discussion
Our study sought to delineate baseline demographic, histopathological and radiological characteristics of patients with KRAS mutant lung cancers. The majority of the patients were male, and 43.6% were smokers. Existing literature suggests a correlation between KRAS mutations and race and smoking status, with patients being more commonly white and current or former smokers. The incidence of smoking in our population was not as high as in some studies, but still higher than the general incidence of smoking in patients with NSCLCs, which is around 15% [14]. Most patients had metastatic disease, with the most common sites of metastases being non-regional lymph nodes, lung, pleural and bone. Adenocarcinoma was the commonest histology, and the majority of tumours were poorly differentiated. The most common type of mutation was G12C, seen in 39.8% patients. This is consistent with data from other studies, which have reported G12C mutation incidence rates of 33%–45% [15–17].
We described radiological characteristics of KRAS mutant lung cancers in our hospital and compared these findings between G12C and non-G12C subtypes. We did not find a statistically significant difference between the groups based on mutation subtype. G12C KRAS mutant lung cancers have been reported to be likely to have a cavitary primary tumour with a higher frequency of lung metastases [18]. Relative to NSCLC with fusion rearrangements, a lower frequency of pleural metastases and lymphangitic carcinomatosis have been reported in G12C KRAS mutant lung cancers; brain and soft tissue metastases are, however, more common. The most common sites of metastases in our cohort were the lung, non-regional lymph nodes and pleura. Brain metastases were present in 12.7% of our patients overall, significantly higher in the G12C group (20.7% versus 7.5%). In a previous study, the most common sites of metastases in KRAS mutant lung cancers were bone, brain and lungs [18]. Yang et al [19] demonstrated that KRAS mutations were risk factors for brain metastasis in male patients with lung adenocarcinomas. The higher incidence of brain metastases in the G12C KRAS subgroup is clinically relevant and suggests these patients may benefit from closer monitoring for the development of neurological signs and/or symptoms and underlines the need for treatment modalities with reliable efficacy and penetration beyond the blood–brain barrier.
KRAS mutations have demonstrated a poorer OS compared to KRAS wild-type NSCLC [20]. In our study, the median PFS with first-line chemotherapy was 6 months, with a median OS of 12 months. This is consistent with previous reports [21–25].
Results on the impact of specific codon and point mutations on survival are variable. In a study of 677 patients with KRAS mutated advance stage NSCLC, those with mutations in KRAS codon 13 (n = 53) had poorer outcomes in comparison to codon 12 (n = 624), with a difference in survival of 2 months (p = 0.008), after adjusting for age, sex and smoking status [26]. Another analysis of 450 patients with KRAS mutated, metastatic adenocarcinoma lung found no OS difference between codon 12 and 13 mutations, although those with codon 13 mutations had a numerically lower 2-year survival [27]. Other studies demonstrated genotypic differences in survival, with G12C and p.G12V mutations being reported to have poorer survival relative to other subtypes [28]. One Asian study of 75 patients with advanced NSCLC reported improved PFS for patients with KRAS p.G12C mutations, particularly when treated with first-line pemetrexed-based chemotherapy [29]. In our population, G12C mutation was associated with inferior PFS and OS, consistent with most previous studies. Brain metastases and PS ≥ 2 were also predictive of poor survival, consistent with existing literature. There are conflicting data regarding the prognostic implication of KRAS mutations in earlier-stage lung cancers [30–32]. The small number of patients in our cohort receiving curative intent therapy precludes analysis.
Our study found no significant survival differences between smokers and non-smokers, although some studies have reported superior OS for never-smokers compared to current or former smokers [33]. This difference may be due to the lack of homogeneity among subgroups, with KRAS mutations being commoner in smokers, and EGFR and ALK mutations more common in non-smokers. When adjusting for the specific mutation, no survival difference between current/former/never smoking status has been demonstrated [34].
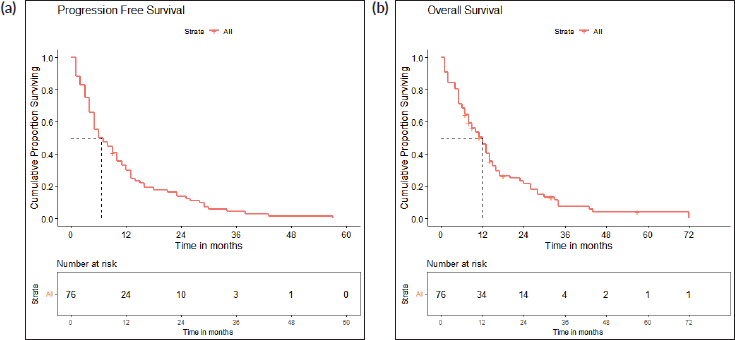
Figure 2. (a): Progression-free survival. A Kaplan-Meier curve depicting the PFS of all patients included in the survival analysis (n = 76). (b): Overall survival. A Kaplan-Meier curve depicting the OS of all patients included in the survival analysis (n = 76).
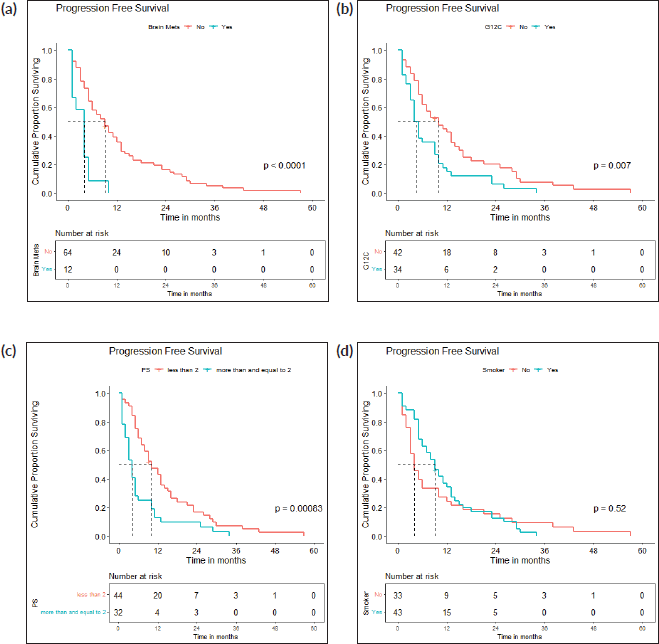
Figure 3. (a): PFS: brain metastases present versus absent. A Kaplan-Meier curve depicting the PFS of patients with (n = 12) and without (n = 64) brain metastases. (b): PFS: g12c versus other mutations. A Kaplan-Meier curve depicting the PFS of patients with (n = 34) and without (n = 42) G12C mutations. (c): PFS: PS <2 versus ≥2. A Kaplan-Meier curve depicting the PFS of patients included with ECOG PS <2 (n = 44) and ≥2 (n = 32). (d): PFS: smokers versus non-smokers. A Kaplan-Meier curve depicting the PFS of smokers (n = 43) and non-smokers (n = 33).
KRAS mutations may coexist with other master genes. The commonest co-occurring mutation in our cohort was TP53 mutation. In a Lung Cancer Mutation Consortium study, an additional carcinogenic driver was identified in a third of patients with KRAS mutations [27]. These mutations may have prognostic implications. Skoulidis et al [35] reported superior objective response rates for NSCLC with co-existent TP53 mutations in comparison with those with KRAS mutations alone (28.6% and 35.7%, respectively).
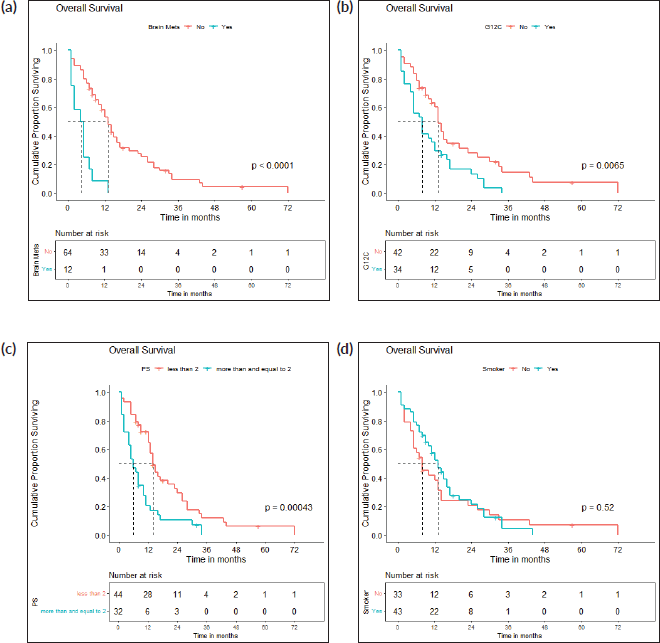
Figure 4. (a): OS: brain metastases present versus absent. A Kaplan-Meier curve depicting the PFS of patients with (n = 12) and without (n = 64) brain metastases. (b): OS: g12c versus other mutations. A Kaplan-Meier curve depicting the OS of patients with (n = 34) and without (n = 42) G12C mutations. (c): OS: PS <2 versus ≥2. A Kaplan-Meier curve depicting the PFS of patients included with ECOG PS <2 (n = 44) and ≥2 (n = 32). (d): OS: smokers versus non-smokers. A Kaplan-Meier curve depicting the OS of smokers (n = 43) and non-smokers (n = 33).
The majority of patients in our study received intravenous chemotherapy, with only a few being offered immunotherapy [36]. The low uptake of immunotherapy in our population is primarily due to financial challenges. The role of immunotherapy in the first-line treatment of patients with KRAS-mutant NSCLC is well established. Blocking the PD-1/PD-L1 pathway has been promising, with randomised phase II and III trials demonstrating improvement of OS in KRAS mutant NSCLC with checkpoint inhibitors compared to standard chemotherapy, as well as a recent meta-analysis reporting an OS improvement with anti-PD-(L)1 agents with or without chemotherapy in KRAS-mutant NSCLC in the first-line setting [37–40]. Authors have hypothesised the potential use of KRAS mutation status as a predictive biomarker in the selection of patients for immune checkpoint inhibitors as no significant OS benefit was observed for patients with KRAS wild type cancers. Due to the small number of patients receiving immunotherapy in our patient population due to financial constraints, a larger population and longer follow-up is needed to establish results.
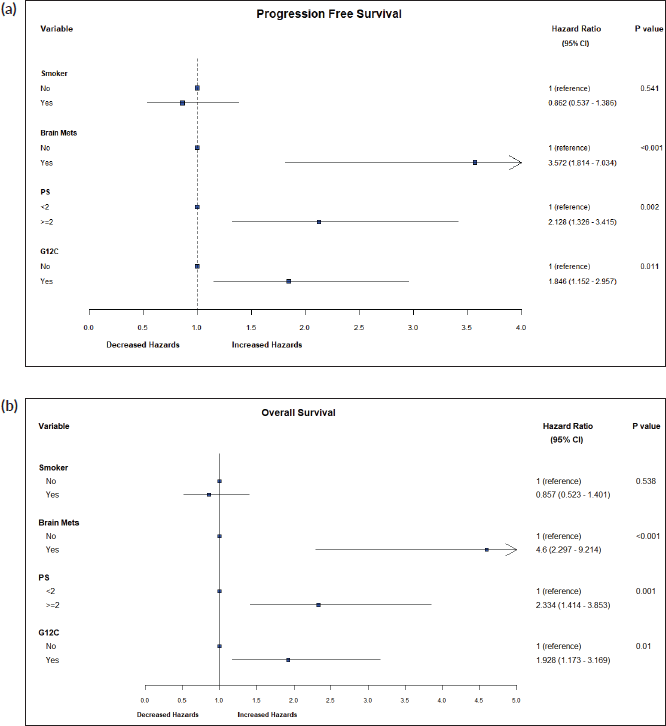
Figure 5. (a): Hazard ratios for PFS. A forest plot of hazard ratios for prognostic variables for PFS. (b): Hazard ratios for OS. A forest plot of hazard ratios for prognostic variables for OS.
Our study is limited by the small sample size, retrospective nature and unavailability of complete treatment records of a considerable number of our subjects. The need remains for appropriately powered studies with larger sample sizes to enhance our understanding of clinical, radiological and pathological characteristics of KRAS mutant lung cancers as well as therapeutic implications.
Conclusion
This is the largest dataset of KRAS mutant lung cancers from India. Nearly half were smokers. 12.7% had brain metastases, significantly more in patients with G12C mutations and were associated with poorer PFS and OS. G12C mutation, the commonest subtype, was associated with inferior PFS, consistent with previous studies. Experience with targeted therapy for KRAS mutations remains an area of future exploration due to the unavailability of these agents in India.
Acknowledgments
We are grateful to our Thoracic Disease Management Group for inputs during clinical decision making in various cases.
Conflicts of interest
There is no conflict of interest to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author contributions
Vanita Noronha: conceptualisation, methodology, data curation, resources and supervision; writing – review and editing; Laboni Sarkar: conceptualisation, methodology and data curation; formal analysis; writing – original draft preparation; and writing – review and editing; Vijay Patil: data curation and resources; Nandini Menon: data curation and resources; Minit Shah: data curation and resources; Akash Pawar: methodology and formal analysis; Oindrila Roy Chowdhury: methodology and formal analysis; Omshree Shetty: data curation and resources; Anuradha Chougule: data curation and resources; Pratik Chandrani: data curation and resources; Rajiv Kaushal: data curation and resources; Trupti Pai: data curation and resources; Amit Janu: data curation and resources; Nivedita Chakrabarty: data curation and resources; and Kumar Prabhash: conceptualisation, methodology, data curation, resources and supervision; writing – review and editing.
References
1. Batra U and Nathany S (2021) Biomarker series: KRAS – a narrative review Cancer Res Stat Treat 4 516–523 https://doi.org/10.4103/crst.crst_11_21
2. Gheware AP, Rathor A, and Jain D (2021) From bench to bedside and beyond: challenges in direct KRAS targeting Cancer Res Stat Treat 4 789–790 https://doi.org/10.4103/crst.crst_248_21
3. Prabhash K, Vora A, and Limaye S, et al (2021) Treatment of advanced non-small-cell lung cancer: first line, maintenance, and second line – Indian consensus statement update (under the aegis of Lung Cancer Consortium Asia, Indian Cooperative Oncology Network, Indian Society of Medical and Pediatric Oncology, Molecular Oncology Society, and Association of Physicians of India) Cancer Res Stat Treat 4 279–314 https://doi.org/10.4103/crst.crst_61_21
4. Skoulidis F, Li BT, and Dy GK, et al (2021) Sotorasib for Lung cancers with KRAS p.G12C mutation N Engl J Med 384 2371–2381 https://doi.org/10.1056/NEJMoa2103695 PMID: 34096690 PMCID: 9116274
5. Agrawal AK, Pragya R, and Choudhary A, et al (2021) Sotorasib – an inhibitor of KRAS p.G12c mutation in advanced non-small cell carcinoma: a narrative drug review Cancer Res Stat Treat 4 524–528
6. Mascaux C, Iannino N, and Martin B, et al (2005) The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis Br J Cancer 92 131–139 https://doi.org/10.1038/sj.bjc.6602258
7. Macerelli M, Caramella C, and Faivre L, et al (2014) Does KRAS mutational status predict chemoresistance in advanced non-small cell lung cancer (NSCLC)? Lung Cancer 83 383–398 https://doi.org/10.1016/j.lungcan.2013.12.013 PMID: 24439569
8. Pao W, Wang TY, and Riely GJ, et al (2005) KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib PLoS Med 2 e17 https://doi.org/10.1371/journal.pmed.0020017 PMID: 15696205 PMCID: 545207
9. Massarelli E, Varella-Garcia M, and Tang X, et al (2007) KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer Clin Cancer Res 13 2890–2896 https://doi.org/10.1158/1078-0432.CCR-06-3043 PMID: 17504988
10. Pawar A, Chowdhury OR, and Salvi O (2022) A narrative review of survival analysis in oncology using R Cancer Res Stat Treat 5 554–561
11. Chakraborty S (2018) A step-wise guide to performing survival analysis Cancer Res Stat Treat 1 41–45
12. Dessai S, Simha V, and Patil V (2018) Stepwise Cox regression analysis in SPSS Cancer Res Stat Treat 1 167–170
13. Dessai S and Patil V (2019) Testing and interpreting assumptions of COX regression analysis Cancer Res Stat Treat 2 108–111 https://doi.org/10.4103/CRST.CRST_40_19
14. Riely GJ, Kris MG, and Rosenbaum D, et al (2008) Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma Clin Cancer Res 14(18) 5731–5734 https://doi.org/10.1158/1078-0432.CCR-08-0646 PMID: 18794081 PMCID: 2754127
15. Nacchio M, Sgariglia R, and Gristina V, et al (2020) KRAS mutations testing in non-small cell lung cancer: the role of Liquid biopsy in the basal setting J Thorac Dis 12(7) 3836–3843 https://doi.org/10.21037/jtd.2020.01.19 PMID: 32802465 PMCID: 7399406
16. Cui W, Franchini F, and Alexander M, et al (2020) Real world outcomes in KRAS G12C mutation positive non-small cell lung cancer Lung Cancer 146 310–317 https://doi.org/10.1016/j.lungcan.2020.06.030 PMID: 32619782
17. Aredo JV, Padda SK, and Kunder CA, et al (2019) Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes Lung Cancer 133 144–150 https://doi.org/10.1016/j.lungcan.2019.05.015 PMID: 31200821 PMCID: 9348589
18. Wu MY, Zhang EW, and Strickland MR, et al (2021) Clinical and imaging features of non-small cell lung cancer with G12C KRAS mutation Cancers (Basel) 13(14) 3572 https://doi.org/10.3390/cancers13143572 PMID: 34298783 PMCID: 8304953
19. Yang B, Lee H, and Um SW, et al (2019) Incidence of brain metastasis in lung adenocarcinoma at initial diagnosis on the basis of stage and genetic alterations Lung Cancer 129 28–34 https://doi.org/10.1016/j.lungcan.2018.12.027 PMID: 30797488
20. Goulding RE, Chenoweth M, and Carter GC, et al (2020) KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: a systematic literature review and meta-analysis Cancer Treat Res Commun 24 100200 https://doi.org/10.1016/j.ctarc.2020.100200 PMID: 32750661
21. Hames ML, Chen H, and Iams W, et al (2016) Correlation between KRAS mutation status and response to chemotherapy in patients with advanced non-small cell lung cancer Lung Cancer 92 29–34 https://doi.org/10.1016/j.lungcan.2015.11.004 PMID: 26775593 PMCID: 4874190
22. Metro G, Chiari R, and Duranti S, et al (2012) Impact of specific mutant KRAS on clinical outcome of EGFR-TKI-treated advanced non-small cell lung cancer patients with an EGFR wild type genotype Lung Cancer 78(1) 81–86 https://doi.org/10.1016/j.lungcan.2012.06.005 PMID: 22770374
23. Fiala O, Pesek M, and Finek J, et al (2013) The dominant role of G12C over other KRAS mutation types in the negative prediction of efficacy of epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer Cancer Genet 206(1–2) 26–31 https://doi.org/10.1016/j.cancergen.2012.12.003 PMID: 23313110
24. Zer A, Ding K, and Lee SM, et al (2016) Pooled analysis of the prognostic and predictive value of KRAS mutation status and mutation subtype in patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors J Thorac Oncol 11(3) 312–323 https://doi.org/10.1016/j.jtho.2015.11.010 PMID: 26749487
25. Dong ZY, Zhong WZ, and Zhang XC, et al (2017) Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma Clin Cancer Res 23(12) 3012–3024 https://doi.org/10.1158/1078-0432.CCR-16-2554 PMID: 28039262
26. Yu HA, Sima CS, and Shen R, et al (2015) Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas J Thorac Oncol 10(3) 431–437 https://doi.org/10.1097/JTO.0000000000000432 PMCID: 4479120
27. El Osta B, Behera M, and Kim S, et al (2019) Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: the lung cancer mutation consortium experience J Thorac Oncol 14(5) 876–889 https://doi.org/10.1016/j.jtho.2019.01.020 PMID: 30735816 PMCID: 8108452
28. Ihle NT, Byers LA, and Kim ES, et al (2012) Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome J Natl Cancer Inst 104 228–239 https://doi.org/10.1093/jnci/djr523 PMID: 22247021 PMCID: 3274509
29. Lei L, Wang WX, and Yu ZY, et al (2020) A real-world study in advanced non-small cell lung cancer with KRAS mutations Transl Oncol 13 329–335 https://doi.org/10.1016/j.tranon.2019.12.004
30. Keohavong P, DeMichele MA, and Melacrinos AC, et al (1996) Detection of KRAS mutations in lung carcinomas: relationship to prognosis Clin Cancer Res 2 411–418 PMID: 9816185
31. Nadal E, Chen G, and Prensner JR, et al (2014) KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma J Thorac Oncol 9 1513–1512 https://doi.org/10.1097/JTO.0000000000000305 PMID: 25170638
32. Izar B, Zhou H, and Heist RS, et al (2014) The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma J Thorac Oncol 9 1363–1369 https://doi.org/10.1097/JTO.0000000000000266 PMID: 25122432
33. Shepherd FA, Domerg C, and Hainaut P, et al (2013) Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy J Clin Oncol 31 2173–2181 https://doi.org/10.1200/JCO.2012.48.1390 PMID: 23630215 PMCID: 4881333
34. Paik PK, Johnson ML, and D’Angelo SP, et al (2012) Driver mutations determine survival in smokers and never-smokers with stage IIIB/IV lung adenocarcinomas Cancer 118(23) 5840–5847 https://doi.org/10.1002/cncr.27637 PMID: 22605530 PMCID: 3424296
35. Skoulidis F, Goldberg ME, and Greenawalt DM, et al (2018) STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma Cancer Discov 8(7) 822–835 https://doi.org/10.1158/2159-8290.CD-18-0099 PMID: 29773717 PMCID: 6030433
36. Menon N and Mailankody S (2018) Immunotherapy protocols in lung cancer Cancer Res Stat Treat 1 139–162
37. Borghaei H, Paz-Ares L, and Horn L, et al (2015) Nivolumab vs docetaxel in advanced nonsquamous cell non-small-cell lung cancer N Engl J Med 373 1627–1639 https://doi.org/10.1056/NEJMoa1507643 PMID: 26412456 PMCID: 5705936
38. Rittmeyer A, Barlesi F, and Waterkamp D, et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open label, multicentre randomised controlled trial Lancet 389 255–265 https://doi.org/10.1016/S0140-6736(16)32517-X
39. Herbst RS, Baas P, and Kim DW, et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1 positive, advanced non-small-cell lung cancer (KEYNOTE010): a randomised controlled trial Lancet 387 1540–1550 https://doi.org/10.1016/S0140-6736(15)01281-7
40. Landre T, Justeau G, and Assié JB, et al (2022) Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials Cancer Immunol Immunother 71(3) 719–726 https://doi.org/10.1007/s00262-021-03031-1






