Cancer histotypes and trends in Azare, Northeast Nigeria: impact of diagnostic support disparity in data reporting
Uchenna Simon Ezenkwa1,a, Mohammed Ibrahim Imam2, Maimuna Orahachi Yusuf3, Abdullahi Sani Giade4, Iragbogie Al-Mustapha Imoudu3, Dauda Abubakar Katagum5 and Bala Muhammad Audu5
1Department of Histopathology, Federal Medical Centre Azare, Bauchi State Nigeria 751101
2Department of Pathology, Bayern University/Aminu Kano University Teaching Hospital, Kano, Nigeria 700233
3Department of Pediatrics, Federal Medical Centre Azare, Bauchi State Nigeria 751101
4Department of Surgery, Federal Medical Centre Azare, Bauchi State Nigeria 751101
5Department of Obstetrics and Gynaecology, College of Medicine, Federal University of Health Sciences, Azare Bauchi State Nigeria 751101
ahttps://orcid.org/0000-0002-7022-8268
Abstract
Background: Definitive, affordable, and timely diagnosis of cancer is key to providing data for surveillance and control programmes. Care disparities have been shown to contribute to poorer survival, especially in resource-constrained populations. Here, we describe the profile of histologically diagnosed cancers in our hospital and highlight the possible effects of inadequate diagnostic support on data reporting.
Methods: We designed a retrospective cross-sectional descriptive study to review histopathology reports archived at the Department of Pathology of our hospital spanning from January 2011 to December 2022. Cases diagnosed as cancer were retrieved and classified by systems, organs and histology types alongside the patient’s age and gender. The trend in the volume of pathology requests and the corresponding malignant diagnosis yield over the period was also documented. Data generated were analyzed statistically using appropriate statistics and presented as proportions and means, with the level of statistical significance set at p < 0.05.
Results: There were 488 cancers out of 3,237 histopathology requests received within the study period. Of these 316 (64.7%) were females. Overall mean age was 48.8 ± 18.6 years with a peak age at the sixth decade, females being significantly younger (46.1 versus 53.5 years; p < 0.001). The top five cancers were breast (22.7%), cervical (12.7%), prostate (11.7%), skin (10.7%) and colorectal cancers (8%). Among females, breast, cervical and ovarian cancers predominated, whereas prostate, skin and colorectal cancers, were commonest among males in decreasing order. Paediatric malignancies accounted for 3.7% of all the cases, most being small round blue cell tumours. The volume of pathology requests rose remarkably from 95 cases in 2014 to 625 cases in 2022 with a corresponding increase in cancer case diagnoses.
Conclusion: Cancer subtypes and ranking in this study are similar to those from urban populations in Nigeria and Africa, despite the low number of cases recorded. Efforts to reduce the disease burden are warranted.
Keywords: cancer histotypes, trends, diagnostic disparity, cancer data reporting, Northeast Nigeria
Correspondence to: Uchenna Simon Ezenkwa
Email: uchennaezenkwa@gmail.com
Published: 24/04/2023
Received: 05/02/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer is a disease generating attention globally owing to the need to stem its rising incidence and improve survival. The GLOBOCAN data estimate that 19.3 million incident cases with 10.0 million deaths were recorded globally in 2020, representing a 7% increase in incidence and a 3% increase in mortality over that recorded in 2018 [1, 2]. Risk factors associated with the development of cancer are largely lifestyle-dependent and vary according to sociodemographic factors [3]. Likewise, the disparity in diagnosis and treatment contributes to poorer survival in low-middle-income countries [4, 5]. Africa accounted for about 6% of the world’s incident cases in 2020 which were derived from cancer registries [6]. Both population and hospital-based data from Nigeria have reported a rising number of cancer cases in Nigeria [7, 8–10]. There is however a dearth of comprehensive data describing the spectrum of malignancies in our immediate environment in the Northeast Nigeria except for a previous report of gynaecological cancers [11]. As our objective, we set to describe cancer histological types, the trends in diagnosis and the influence diagnostic support availability could have on data reporting. Understanding our situation will provide invaluable data for awareness propagation and risk-modifying strategies in addition to contributing to the growing knowledge base on cancer among Nigerians living in our locale.
Materials and methods
This retrospective cross-sectional descriptive study was conducted at the Federal Medical Centre Azare, Bauchi State Northeast Nigeria. Azare is situated at latitude 11°40′27ʺ North and longitude 10°11′28ʺ East with an estimated population of about 110,452 using the 2007 census figures, the inhabitants being mostly agricultural in occupation consisting of pastoral and crop farming [12]. The hospital is a 350-bed capacity tertiary health institution and serves as a referral centre for all the primary and secondary health care centres in the communities and towns from neighbouring states, while the two closest tertiary hospitals with histopathological services were located about 200 km away each. Histopathology reports archived at the Department of Pathology of our hospital from January 2011 to December 2022 were reviewed.
The majority of requests (about 75%) were derived from primary hospital patients who accessed healthcare directly while fewer patients were referred from primary and secondary health centres for further management or investigation. The average cost of histopathology testing over this period was N1,500 (N1,200 to N2,800), and available oncology services were mainly surgical excision with curative intent and chemotherapy. For this study, samples reported as cancers were retrieved with the documented patient age, gender and cancer site, and classified into systems, organs and histology types. The trend in annual case requests and cancer diagnosis was additionally determined.
The Statistical Package for Social Sciences version 20 was used to analyse the data generated. Descriptive statistics was applied to the data to determine the proportions of variables. The difference in means between continuous variables was also analysed using the student t-test function. The results were presented in prose, figures and tables.
All patient data were fully deidentified prior to analysis. This study adhered to the Helsinki Declaration on Research on human subjects of 1964 and its later amendments. Institutional review board approval was received from the hospital’s ethics committee prior to the commencement of the study.
Results
There were 3,237 histopathological requests within the time period. A total of 488 cancers were diagnosed histologically during this period representing 15.1% of all received histological specimens. Of these malignant cases, there were 316 females and 172 males giving a female-to-male ratio of 1.8:1. Overall mean age was 48.8 ± 18.6 years, females being significantly younger (46.1 versus 53.5 years; p < 0.001). Age group by decades of the patients is presented in Figure 1 with the peak age being in the sixth decade.
Table 1 shows the list of all cancers documented. Overall, the top five cancers were breast (22.7%), cervical (12.7%), prostate (11.7%), skin (10.7%) and colorectal cancers (8.0%). There were cases of metastatic cancers whose primary sites were not resolved. These were less than 1% of the cases. Among females, breast, cervix and ovarian cancers predominated, while prostate, skin, and colorectal cancers, were commonest among males in descending order (Tables 2 and 3). Gallbladder, soft tissue, skin and sinonasal cancers were about equal in both genders. Paediatric malignancies accounted for 3.7% of the cases, most being small round blue cell tumours.
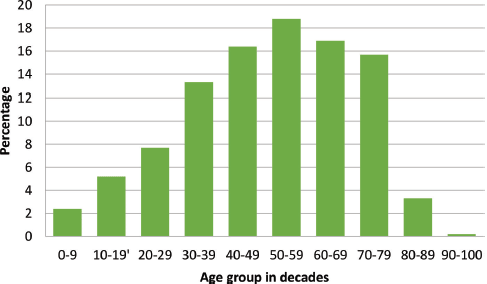
Figure 1. Age group of cancer patients in decades with a progressive increase in cancer occurrence as age advances.
Table 1. Cancer types by organs in decreasing frequency and percentage for both gender and all ages.
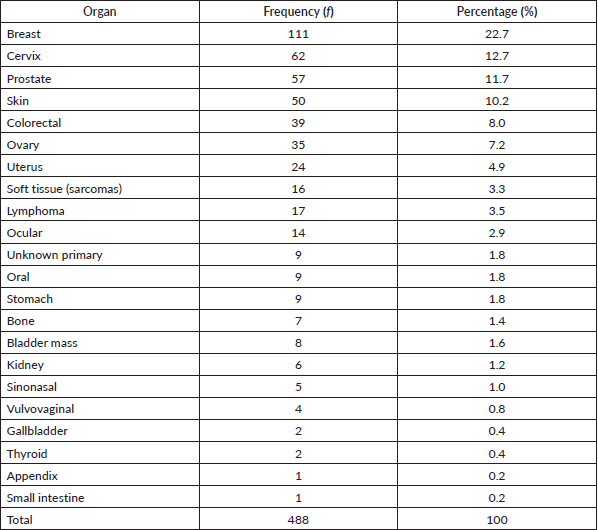
Table 2. Female cancers by organ, frequency and percentage.
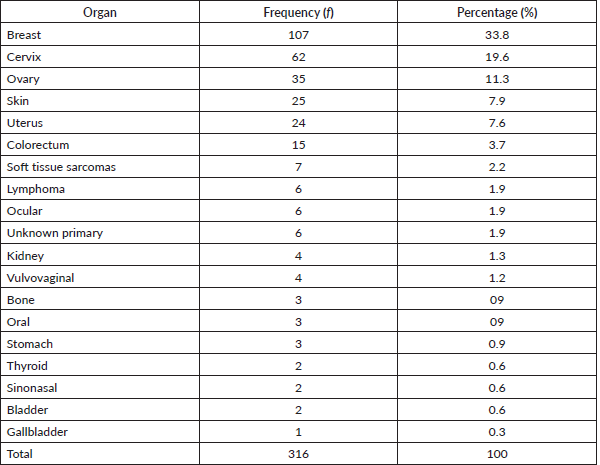
Table 3. Male cancers by organ, their frequency and percentage of the occurrence.
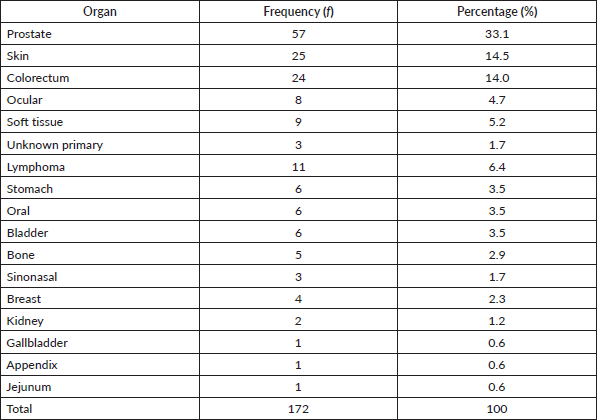
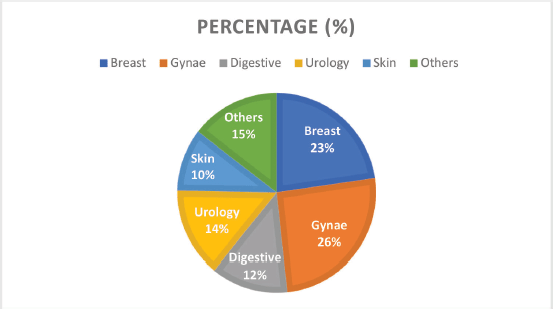
Figure 2. Cancer types by the system and their frequencies.
Grouped according to systems, gynaecological cancers were the commonest, accounting for about 26% of all the cases (Figure 2). Each system group had different histological types of cancers. Figure 3 shows a photomicrographic representation of the top five cancers in both genders combined as seen in this study.
Cancer by system
The malignancies seen here were further classified according to the organ or system involved and the most common five groups are presented in more detail below. These groups include the gynaecological, breast (mammary), digestive, urological and skin (dermatological) cancers. In addition, paediatric cancers are also described to highlight the spectrum of malignant tumours with similar phenotypes but occurring in different organs and systems of the body.
Gynaecological cancers
This was the commonest system involved in cancer accounting for 125 (25.6%) of all cases. They were comprised of ovarian, uterine, cervical and vulvovaginal cancers. The median age of these patients was 50 years ranging from 17 to 75 years. Over half of the cases occurred in the fifth to seventh decades of life.
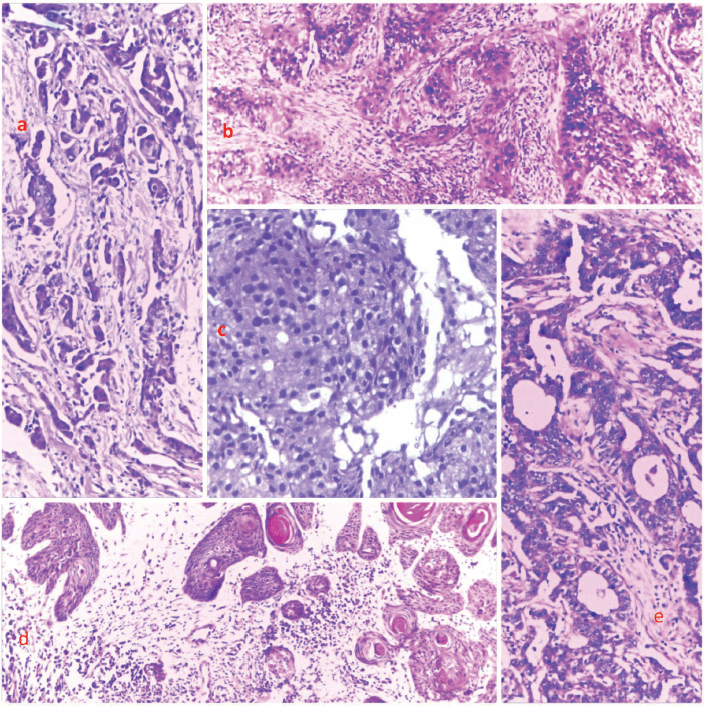
Figure 3. Photomicrographs showing the histological features of selected cases from the top five cancers. (a): Breast cancer, (b): cervical cancer, (c): prostate cancer, (d): skin cancer and (e): and colon cancer, respectively. H&E, (a): ×40, (b): ×100, (c): ×400, (d): ×40 and (e): ×100.
Cervical cancer was the most predominant in this category accounting for 62 (49.6%) cases. The majority of these were squamous cell carcinoma seen in 51 (85%) of persons, while adenosquamous and adenocarcinomas account for three cases each. Two cases of clear cell carcinoma and one case of sarcoma were also documented.
Thirty-four (28.1%) of gynaecological cancers were from the ovary. The majority (31 out of 35) of these were cystadenocarcinomas out of which, 7 were mucinous varieties. There were also two cases of dysgerminoma and one each of yolk sac tumour and malignant Brenner’s tumour.
Uterine endometrial and myometrial cancers were 23 in number out of which were 14 endometrioid carcinomas, 4 choriocarcinomas, 3 squamous cell carcinomas, 2 leiomyosarcomas and 1 endometrial stromal sarcoma.
There were four cases of vulvovaginal cancers, two of which were choriocarcinomas, and one each of rhabdomyosarcoma and squamous cell carcinoma.
Breast cancer
Breast cancer was the commonest type of cancer from a single body organ (111 cases). About 96.4% occurred in women; 4 (3.6%) cases were seen in men. The median age was 45 years (18–80 years) with a peak in the fifth decade. About 97.3% of the cases were histologically invasive carcinoma of no special type. Other variants observed were secretory carcinoma, sebaceous carcinoma and non-Hodgkin lymphoma and these accounted for 0.9% of all breast cancers each. Laterality was about equal between the right and left breast both having 40.2% and 41.2% each. One patient had bilateral breast cancers while laterality was not documented in about 18 cases.
Urological cancers
Cancers of the kidneys, urinary bladder and prostate are presented in Table 1. The renal malignancies were seen more in females (four out of six), with five cases being renal cell carcinoma and one documented as nephroblastoma histologically. Six of the eight cancers of the urinary bladder were seen in men; these cancers were classified as two squamous cell carcinomas and six urothelial carcinomas. All 57 cases of prostate cancers were adenocarcinomas. Overall, there were 71 urological cancers recorded.
Digestive system cancers
Sixty-one cases were cancers involving the digestive system comprising malignancies of the oral cavity, salivary glands, stomach, biliary, colon and anorectum. These were derived from patients aged 6–90 years with a median of 50 years. The overall peak age was in the age group 50–59 years and most were seen in men with a ratio of 1.6:1.
There were nine cancers of the oral cavity; five of these were in the salivary glands (four parotid and one minor salivary glands), classified histologically as mucoepidermoid carcinomas in three cases and adenoid cystic carcinomas in two cases. The remaining four oral cancers were poorly differentiated in three variants while one was a high-grade sarcoma, and these were distributed two cases each in the palate and the oropharynx.
The gastric cancers seen were all adenocarcinomas and occurred in six males and three females. Three of these patients were in the fourth decade of life, two in the seventh while four patients had age distribution between these two.
Two gallbladder adenocarcinomas were recorded, one in each gender and both patients being in their early sixties. There was one case each of jejunal and appendix cancer, 25 colonic and 14 rectal cancers. Their histology varied, two being neuroendocrine carcinomas, one non-Hodgkin lymphoma while the others were adenocarcinomas. There were more males with a little over a quarter of the patients being less than 50 years of age at the time of diagnosis.
Skin cancers
There were 50 cases of skin cancer with slight female preponderance (female/male ratio 1.1:1) and a median age of 51 years (range 13–80 years). Cutaneous melanoma ranked second with 12 (25%) cases after squamous cell carcinoma which had 29 (56%) cases among all the observed cancers. Three cases of dermatofibrosarcoma protuberance and one of basal cell carcinoma in addition to five undifferentiated carcinomas were also documented. About 60% of these tumours occurred on the lower limb (thigh, leg, foot).
Paediatric cancers
Paediatric cancers were seen in different organs listed in Tables 1–3. As presented in Table 4 below, there were 19 cases derived from 12 males and 7 females with a median age of 8.5 years (range 4–7 years). Cancer with the highest proportion was lymphoma; others were rhabdomyosarcoma, nephroblastoma, retinoblastoma and squamous cell carcinoma on the hand. Additionally, there were two cases described as small round blue cell tumours and one case of skin nodules with epithelial features but not further characterised.
Cancer volume by year and trend over the study period
The overall histopathology request volume seen each year and the trend over the years are shown in Figure 4. Observably, the early part of the curve had fewer number of requests averaging around 200 documented cases with a steady rise in the later years. Expectedly, the number of diagnosed cancer cases reflected the volume of requests proportionately.
Table 4. Paediatric cancer by histologic type.

Figure 4. Trends of histopathological requests and cancer diagnosis over the reviewed period. The least annual volume was in 2014 while a steady increase in both requests and cases was seen from 2019 onwards.
Discussion
The cancer burden is increasingly reported in developing communities transitioning to more Westernised lifestyles [2]. Our study has rather shown low numbers of cancer cases from all body sites seen over a decade in our population. In Africa, Nigeria ranks second after Egypt with the highest number of cancer cases and deaths [6]. There are few population-based cancer registries in Africa, hence, actual cancer incidence and mortality in the population may be under-represented [13]. It is projected that by the end of the present decade, cancer mortality could rise to about a million in the continent [14]. Most of these deaths may be unaccounted for as a result of inadequate data coverage of remote underserved communities such as ours. The present study not only adds to the existing data from our country but further highlights the particular challenges being faced in underserved localities in establishing the true burden of this disease.
Although hospital and microscopy based, the tumour histotypes and their ranking documented here compare favourably with results derived from cancer registries from Nigeria [15]. Indeed, there is a similarity between our top five cancers and those seen in the population-based cancer registries in Nigeria, Africa and globally [2, 6, 7]. The index population is less urban compared to those with cancer registries cited previously and the occupation is mainly agriculture-based, suggesting a less sedentary lifestyle and diets likely high in whole grains and fibre. We think that the effect of these well-known risk factors of carcinogenesis, in addition to obesity and smoking, may not completely explain the similar cancer profiles between our community and those in more developed societies [3]. There is, therefore, a need to investigate probable environmental, cultural or genetic factors driving these tumours in our locality. Also, the absence of lung cancer in the top five seen in Africa and Nigeria may indicate its lower prevalence in the African population or underdiagnosis of these cases [6, 7, 16]. The role of unsafe sexual practices on the high proportion of cervical cancer seen may need validation [3].
The prevailing higher number of breast and gynaecological cancers explains the female preponderance in the report. This is in concordance with Africa continent data but contrary to the global outlook where more males had higher incidence rates in 2020 [2, 6]. Mohammed et al [17], in a report from Kano, a neighbouring state to this study site, showed a slight male preponderance while that by Sahabi and Abdullahi [18], from Sokoto Nigeria had more females. These studies, also based solely on histologically diagnosed cases, likewise had more gynaecological and breast cancers [17, 18]. These variations signify that besides gender-based tumours, most other malignancies were more prevalent among males as was also found in the index study.
The overall mean age of the patients in the present study was less than 50 years suggesting a preponderance among the younger population, similar to a study by Uchendu [8] from the southern part of the country; in addition, we found a significantly younger female population due to higher proportions of female gender-related malignancies. Perhaps this could be ascribed to the prevalent young age of the population. Cancer in the young is indeed assuming alarming proportions the world-over [19]. The increase in observed cases as age increased is in tandem with the established knowledge that cancer incidence increases with age and this is true in every population [20]. However, depending on a population’s life expectancy, more cases may appear prevalent at an either older or younger age. It may therefore be expected that with an increase in life expectancy, the age of our demographics may tilt towards the older age group. In this regard, as more individuals survive infectious and cardiovascular diseases, there will be more people living in old age, and this, combined with the adoption of cancer-promoting lifestyles, may cause more cases of cancer to emerge [20, 21].
The low number of documented cancer cases in our report could be a reflection of the difficulties encountered in navigating the diagnostic pathway, while the trend in the volume of histopathological requests represents periods when the centre had minimal surgical pathology support in the earlier years transitioning to later years with established substantial histopathological workflow, and not due to increase in disease incidence. This shows that having a functioning pathology laboratory is vital in generating good quality data for intervention policy formulation because where this is lacking, the true burden of the disease will be obscured and this is evident in poorer underserved communities in Africa [13, 22]. And when these facilities are located far from the patients, as was the case in our experience, patronage becomes difficult, especially when the cost is borne by the patients [23]. Other authors from Nigeria, even within cities, have noted a negative impact of point-of-care distance on diagnostic service uptake [17]. In effect, these factors further worsen the disparity existing between rural and urban dwellers in cancer care with associated poorer outcomes among the rural population [4, 24].
Certain cancer types such as liver, lung and brain cancers were not represented in this study, perhaps due to the inability to biopsy or resect these tumours. We also observed tumours with unknown primary anatomical sites that were not further investigated owing to a lack of ancillary testing support and this has implications for determining what treatment the patient should receive. Besides its effect on care, tissues and data for genomic research into these malignancies from our environment to understand their characteristics will be lacking. Therefore, building capacity for adequate patient care in such settings should include training skilled oncology personnel for all levels of care in addition to creating the setting for cutting-edge research to prevent, detect or treat these cancers [25–28]. Also, immunohistochemistry and molecular testing of tumours should begin to be prioritised in poorer communities in order to reasonably identify each tumour type and determine predictive and prognostic features for individual patients [29, 30].
Despite the limitations occasioned by the histopathological basis of our data, the percentage of malignant diseases among our received samples (15.1%) did not remarkably differ from others reported in the country with proportions ranging from 13.7% to 24.4% in various local studies [8, 31–33]. The similarities in patients’ demographics, tumour histotypes and profiles between these studies and ours may suggest a similar cancer burden too, even though these other studies had higher volumes of surgical specimens and were derived from more urban cities. A second limitation is the absence of data on tumour stage at diagnosis that was not overcome due to the retrospective nature of the study. However, the presence of few cases of suspected metastatic diseases, without known primary site of origin, shows that there were cases with very advanced disease stages, further suggesting a need to diagnose oncology diseases early and appropriately stage them for therapeutic and prognostic purposes. Enhancing screening services at the primary healthcare level to detect premalignant lesions and early-stage cancers will help in changing this situation. Also, establishing a cancer registry within the hospital with a mandate to follow up with patients even at the community level will help make survival data available for proper prognostic interrogation of this disease.
Conclusion
The cancer disease burden in our population appears low, however, it shares similar histological profiles when compared to other indigenous and non-indigenous data which may suggest under-reporting in our environment given that laboratory diagnostic support is inadequate. This might be masking a silent epidemic that could overwhelm an already weak health system if this trend is not reversed. Dedicated efforts are therefore required to set up responsive oncology services that will enable underserved communities access to quality cancer care and provide data for policymaking, novel adaptable intervention programmes and translational research to eliminate some, if not all the cancer types from the population.
Acknowledgment
We thank Amina Opeyemi Muhammed for helping with the archiving of the data which enabled this study greatly. All the staff of the Pathology department and the surgical teams that worked hard in all the years to ensure our patients got available care are pleasantly appreciated.
Conflicts of interest
The authors declare that they have no conflict of interest to declare with regard to this study.
Source of funding
None.
Institutional review
This study was reviewed and approved by the Federal Medical Centre Azare Ethics, Research and Review Committee before the commencement of the study.
Author contribution
USE, BMA conceived the study. USE, MII retrieved and reviewed the histopathological classification of the cases. USE, MII, IAI, MOY, ASG reviewed the data and statistical analysis. USE, MOY, IAI produced the first manuscript. DAK, BMA reviewed the manuscript extensively. All the authors read and approved the final submission.
References
1. Bray F, Ferlay J, and Soerjomataram I, et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68 394–424 https://doi.org/10.3322/caac.21492 PMID: 30207593
2. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
3. GDB 2019 Cancer Risk Factors Collaborators (2022) The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019 Lancet 400 563–591 https://doi.org/10.1016/S0140-6736(22)01438-6
4. Bergin RJ, Emery J, and Bollard RC, et al (2018) Rural–urban disparities in time to diagnosis and treatment for colorectal and breast cancer Cancer Epidemiol Biomarkers Prev 27(9) 1036–1046 https://doi.org/10.1158/1055-9965.EPI-18-0210 PMID: 29987098
5. Patel MI, Lopez AM, and Blackstock W, et al (2020) Cancer disparities and health equity: a policy statement from the American Society of Clinical Oncology [abstract] J Clin Oncol 38(29) 3439–3449 https://doi.org/10.1200/JCO.20.00642 PMID: 32783672 PMCID: 7527158
6. Sharma R, Nanda M, and Fronterre C, et al (2022) Mapping cancer in Africa: a comprehensive and comparable characterization of 34 cancer types using estimates from GLOBOCAN Front Public Health 10 1–14 https://doi.org/10.3389/fpubh.2022.839835
7. Jedy-Agbaa E, Curado MP, and Ogunbiyi O, et al (2012) Cancer incidence in Nigeria: a report from population-based cancer registries Cancer Epidemiol 36(5) e271–e278 https://doi.org/10.1016/j.canep.2012.04.007
8. Uchendu OJ (2020) Cancer incidence in Nigeria: a tertiary hospital experience Asian Pac J Cancer Care 5(1) 27–32 https://doi.org/10.31557/apjcc.2020.5.1.27-32
9. Awodele O, Adeyomoye AA, and Awodele DF (2015) Cancer distribution pattern in south-western Nigeria Tanzania J Heal Res 13(2) 1–7
10. Saibu M, James A, and Adu OB, et al (2017) Epidemiology and incidence of common cancers in Nigeria J Cancer Biol Res 5(3) 1–7
11. Aminu MB, Ibrahim SM, and Garba NA, et al (2020) Gynaecological malignancies in Azare, Northeast Nigeria: an assessment of types, stage at presentation and treatment affordability Int J Reprod Contracept Obstet Gynecol 9(5) 1895–1899 https://doi.org/10.18203/2320-1770.ijrcog20201776
12. Abubakar A and Joshua T (2022) Bacteriological assessment on ready-to-eat salad sold in some fast food centers in Azare town, Bauchi State, Nigeria Gadaau J Pure Alli Sci 1(1) 68–72 https://doi.org/10.54117/gjpas.v1i1.23
13. Allemani C, Matsuda M, and Di Carlo V, et al (2018) Global surveillance of trends in cancer survival: analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers during 2000–2014 from 322 population-based registries in 71 countries (CONCORD-3) Lancet 391(10125) 1023–1075 https://doi.org/10.1016/S0140-6736(17)33326-3 PMID: 29395269 PMCID: 5879496
14. Sylla BS and Wild CP (2012) A million africans a year dying from cancer by 2030: what can cancer research and control offer to the continent? Int J Cancer 130(2) 245–250 https://doi.org/10.1002/ijc.26333
15. Jemal A, Bray F, and Korir AR, et al (2019) Cancer in Sub-Saharan Africa vol III (International Agency for Research on Cancer)
16. Okonta KE, Echieh PC, and Abubakar U, et al (2021) Management of lung cancer in Africa: underdiagnosis and poor access to treatment – a close look at Nigeria and West African Sub-region J Pan African Thorac Soc 2(3) 122–129 https://doi.org/10.25259/JPATS_11_2021
17. Mohammed AZ, Edino ST, and Ochicha O, et al (2008) Cancer in Nigeria: a 10-year analysis of the Kano Cancer Registry Niger J Med 17(3) 280–284 https://doi.org/10.4314/njm.v17i3.37396 PMID: 18788253
18. Sahabi SM and Abdullahi K (2017) Epidemiological survey of malignant neoplasms in Sokoto, Nigeria World J Res Rev 4(4) 10–15
19. Wen YF, Chen MX, and Yin G, et al (2021) The global, regional, and national burden of cancer among adolescents and young adults in 204 countries and territories, 1990–2019: a population‑based study J Hematol Oncol 14 1–14 https://doi.org/10.1186/s13045-021-01093-3
20. Smetana KJ, Lacina L, and Szabo P, et al (2016) Ageing as an important risk factor for cancer Anticancer Res 36 5009–5017 https://doi.org/10.21873/anticanres.11069 PMID: 27798859
21. Bray F, Laversanne M, and Weiderpass E, et al (2021) The everincreasing importance of cancer as a leading cause of premature death worldwide Cancer 127 3029–3030 https://doi.org/10.1002/cncr.33587 PMID: 34086348
22. Anyigba CA, Awandare GA, and Paemka L (2021) Breast cancer in sub-Saharan Africa: the current state and uncertain future Exp Biol Med 246 1377–1387 https://doi.org/10.1177/15353702211006047
23. Aregbeshola BS and Khan SM (2018) Out-of-pocket payments, catastrophic health expenditure and poverty among households in Nigeria Int J Heal Policy Manag 7(9) 798–806 https://doi.org/10.15171/ijhpm.2018.19
24. Foerster M, Schüz J, and Mckenzie F, et al (2020) Dissecting the journey to breast cancer diagnosis in sub-Saharan Africa: findings from the multicountry ABC-DO cohort study Cancer Epidemiol 148 340–351
25. Sivaram S, Perkins S, and He M, et al (2021) Building capacity for global cancer research: existing opportunities and future directions J Cancer Educ 36(Suppl 1) S5–S24 https://doi.org/10.1007/s13187-021-02043-w
26. Pramesh CS, Badwe RA, and Bhoopathy N, et al (2022) Priorities for cancer research in low- and middle-income countries: a global perspective Nat Med 28 649–657 https://doi.org/10.1038/s41591-022-01738-x PMID: 35440716 PMCID: 9108683
27. Sullivan R, Alatise OI, and Anderson BO, et al (2015) Global cancer surgery: delivering safe, aff ordable, and timely cancer surgery Lancet Oncol 16 1193–1224 https://doi.org/10.1016/S1470-2045(15)00223-5 PMID: 26427363
28. Are C, Mcmasters KM, and Giuliano A, et al (2019) Global forum of cancer surgeons: perspectives on barriers to surgical care for cancer patients and potential solutions Ann Surg Oncol [Internet] 26(6) 1577–1582 https://doi.org/10.1245/s10434-019-07301-2 PMID: 30911947
29. Ziegenhorn H, Frie KG, and Ekanem I, et al (2020) Breast cancer pathology services in sub-Saharan Africa: a survey within population-based cancer registries BMC Health Serv Res 20 1–9 https://doi.org/10.1186/s12913-020-05752-y
30. Nass SJ, Cohen MB, and Nayar R, et al (2019) Improving cancer diagnosis and care: patient access to high-quality oncologic pathology Oncologist 24 1287–1290 https://doi.org/10.1634/theoncologist.2019-0261 PMID: 31366725 PMCID: 6795152
31. Yusuf I, Atanda AT, and Umar AB, et al (2017) Cancer in Kano, Northwestern Nigeria: a 10‑year update of the Kano Cancer Registry Ann Trop Pathol 8 87–93
32. Mandong BM, Madaki AKJ, and Mannaseh AN (2003) Malignant disease in Jos: a follw up Ann Afr Med 2(2) 49–53
33. Nwafor CC and Nwafor NN (2018) The pattern and distribution of cancers in Akwa Ibom State, Nigeria Niger J Clin Pract 21 603–608 https://doi.org/10.4103/njcp.njcp_316_17 PMID: 29735861






