Experience of bipolar androgen therapy (BAT) in Argentinian oncology centres
Martín Zarbá1†,a, Martin Angel2†,b, Federico Losco2,c, Juan José Zarbá3,d, Juan Carlos Pupilli4, Matías Rodrigo Chacon5,e and Juan Pablo Sade2,f
1FUCA, Instituto Alexander Fleming, CABA C1426ANZ, Argentina
2Genitourinary Tumors Department, Instituto Alexander Fleming, CABA C1426ANZ, Argentina
3Oncology Department, Hospital Zenon Santillan, San Miguel de Tucuman T4000IAK, Argentina
4Genitourinary Tumors Department, Sanatorio Británico Rosario, Santa Fé S2000ANZ, Argentina
5Oncology Department, Instituto Alexander Fleming, CABA C1426ANZ, Argentina
†These authors have contributed equally to this work.
ahttps://orcid.org/0000-0003-3642-4035
bhttps://orcid.org/0000-0002-1463-8887
chttps://orcid.org/0000-0001-5084-3012
dhttps://orcid.org/0000-0003-1013-3993
ehttps://orcid.org/0000-0001-6872-4185
fhttps://orcid.org/0000-0001-9312-5280
Abstract
Background: Previous studies with bipolar androgen therapy (BAT) have shown clinical activity in metastatic Castration Resistant Prostate Cancer (mCRPC) as well as the potential to re-sensitise prostate cancer cells to prior androgen receptor-targeted agents. None of these studies had tested BAT after chemotherapy. In this study, we gathered real-world evidence from three centres in Argentina where BAT is being used in castration-resistant prostate cancer (CRPC), not only prior to chemotherapy but also after several lines of treatment.
Materials and methods: This retro-prospective nonrandomised multicentre cohort study included patients with mCRPC, who received BAT in different scenarios defined by the treating physician at three centres in Argentina.
Results: A total of 21 asymptomatic patients with mCRPC were included. There was a median of two lines before BAT, with nine patients (42.8%) receiving three or more lines, and 13 patients (61.9%) receiving chemotherapy previously. Previous lines included next-generation hormonal agents (NHA) in 100% (abiraterone 33.3% and enzalutamide 71.4%), chemotherapy in 61.9%, Radium-223 in 47.6% and others in 4.8%. The progression free survival (PFS) after BAT was 3.5 months (95% CI: 3.06–7.97). PSA50 response rate (RR) was 28.5% and the overall RR was 14.3%. Of the 17 patients who had disease progression, 9 had a rechallenge to NHA, achieving a 55% RR, 6 received other treatment (chemotherapy in 5 and 177Lu-PSMA in 1) with a 66% RR and 2 best supportive care. The PFS2, calculated after the initiation of BAT in the 15 patients who received further therapy, was 7.93 months (95% CI: 6.73–NR). Treatment was overall well tolerated, with only two patients requiring hospitalisation and treatment interruption due to worsening pain.
Conclusion: To the authors’ knowledge, this is the first publication of BAT in later lines of therapy in mCRPC. BAT showed clinical activity in this scenario. Our data supports that BAT may play a role in CRPC re-sensitisation after multiple treatment lines.
Keywords: androgen deprivation, castration resistance, supraphysiological testosterone
Correspondence to: Martin Angel
Email: mangel@alexanderfleming.org
Published: 02/12/2022
Received: 05/09/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Throughout the entire course of the disease, prostate cancer cells are dependent on the androgen receptor (AR) signalling [1]. Therefore, androgen deprivation therapy (ADT) is a highly effective treatment for this illness and has remained as the backbone of care since Huggins and Hodges [2] research in the 40s. However, ADT is not curative, and practically all patients will eventually experience disease progression to a castration-resistant prostate cancer (CRPC) [3]. Even then, ADT should not be suspended, as prostate cancer cells are still addicted to AR signalling and there is a survival benefit with continued testicular androgen suppression [4].
Resistance to ADT is explained primarily due to molecular adaptations within the AR axis, such as AR overexpression, gene amplification, gain of function point mutations and splice variants of the receptor that lead to transcriptional activity even with the loss of its ligand-binding domain. The result of these adaptive changes is an increase in AR that maintains its activity despite castration levels of testosterone [5, 6]. As the Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer (CARD) trial showed, in agreement with other studies, a second androgen signalling targeted inhibitor shows poor outcomes, probably due to the fact that these agents target the same pathway and share common mechanisms of resistance [7–11].
Even though these AR changes produce resistance to ADT, they also produce a liability that can be exploited therapeutically. Experimental data suggested that rapid cycling between supraphysiologic (>1,500 ng/dL) and castrate (<50 ng/dL) testosterone levels (bipolar androgen therapy (BAT)) may re-sensitise CRPC to further androgen-directed therapies [12, 13].
Even though BAT is a promising strategy for the treatment of CRPC and there is growing evidence to support its use, its indication is still not clear, mainly because different trials have tested it in different clinical scenarios, and, to this date, it is still absent in major guidelines [14, 15]. Real-life evidence is scarce and there are no reports found in the literature of BAT in patients with CRPC after chemotherapy [16].
In this study, we aimed to report real-world evidence from different cancer centres in Argentina where BAT is being used in CRPC, not only prior to chemotherapy but also after several lines of treatment.
Materials and methods
Study population and treatment characteristics
This retro-prospective nonrandomised multicentre cohort study included patients seen between 1 January 2017, and 28 February 2022, with a histologically confirmed diagnosis of prostate cancer, in the scenario of castration resistance, who received BAT at any point of the evolution of the disease at three cancer centres in Argentina. Cancer centres are geographically distributed among the country and also have different insurance coverage (private and public patients).
BAT consisted of monthly intramuscular testosterone cypionate (500 mg every 4 weeks) in addition to ongoing luteinising hormone-releasing hormone agonist therapy. Patients were treated until disease progression or unacceptable toxicity. Demographic and clinicopathological characteristics, including age, Eastern Cooperative Oncology Group performance status (ECOG-PS), Gleason score, prostate specific antigen (PSA) levels, comorbidities and previous treatments were collected from medical charts and entered into a predefined centralised database. Safety information was also retrieved, and subsequent treatment strategies, responses, adverse events and discontinuation were also documented.
Disease progression dates and treatment responses were collected from medical charts. Treatment response was evaluated by the investigator-assessed 50% declines in PSA concentration from baseline (PSA50) and by computed tomography or nuclear medicine bone scans in accordance with Prostate Cancer Working Group 3.
Progression-free survival 1 (PFS1) is defined as the time from the first injection of testosterone cypionate to disease progression or death from any cause and PFS2 as the time from the first injection of testosterone cypionate to second objective disease progression or death from any cause, whichever first.
All the patients granted written informed consent to collect medical information from institutional records.
Adverse events were graded as they were registered by the treating physician and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) when this information was missing.
Statistical analysis
Data were summarised as frequencies and percentages for categorical variables and as medians, ranges and interquartile ranges for continuous variables. Data were censored at the last follow-up if the patient was alive. Survival curves were generated using the Kaplan–Meier method. All statistical analyses were performed using Statistical Package for the Social Sciences software version 23.0 (SPSS, Inc., Armonk, NY, USA).
Results
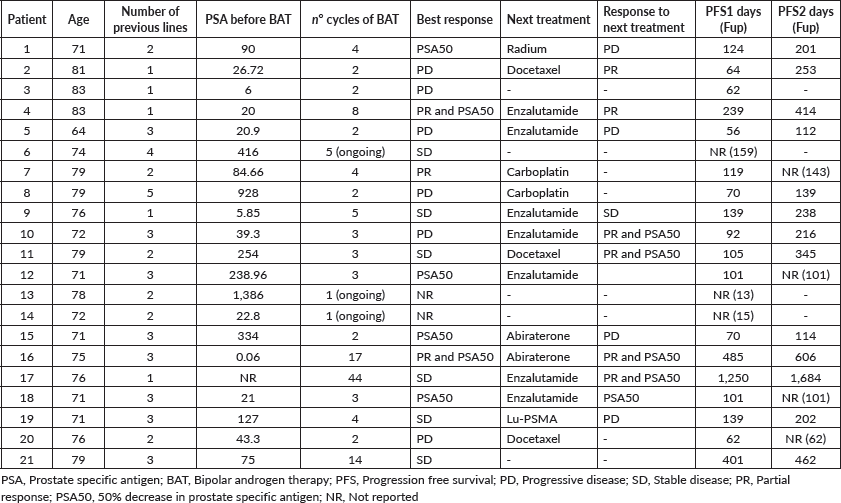
In this multicentre retro-prospective study, a total of 21 patients with mCRPC were included from three Argentinian cancer centres. All patients received BAT in the castrate-resistant scenario at any line of treatment, independent of previous lines. The median age was 75 years (range: 64–83), most patients had an ECOG-PS of 0–1 (90%), although one patient had an ECOG-PS of 3. None of the patients received BAT as first-line treatment of CRPC, with a median of two previous lines, nine patients (42.8%) receiving more than two and one patient (4.7%) receiving five lines of treatments previously. Previous lines included next-generation hormonal agents (NHA) in 100% (abiraterone 33.3% and enzalutamide 71.4%), chemotherapy (docetaxel or cabazitaxel) in 61.9%, Radium-223 in 47.6% and poly (ADP-ribose) polymerase inhibitors in 4.7% (in context of a clinical trial). Baseline characteristics of all the patients are summarised in Table 1.
The cases included are summarised in Table 2.
After a median follow-up of 6.7 months (95% CI: 3.7–11.5), 17 progression events occurred. The median PFS1 was 3.5 months (95% CI: 3.06–7.97) (Figure 1a). PSA50 response rate (RR) was 28.5% and the overall RR was 14.3% (Figure 2). Of the 17 patients who progressed to BAT, 15 (88.2%) received another line of treatment, and 9 of them (52.9%) had a rechallenge to prior NHA. Response, either clinical or biochemical, was achieved in 5 (55%) of patients who were retreated with NHA. Of the five patients who responded, four were rechallenged with enzalutamide, and one with abiraterone; three after one line and the other two after three lines of treatment. The median PFS2, in the 15 patients who received further therapy, was 7.93 months (95% CI: 6.73–NR) (Figure 1b).
Treatment was overall well tolerated. Most patients were asymptomatic at time of BAT initiation, only two patients presented with grade 1 bone pain at baseline. Main adverse events were grade 3 (worsening of pain) pain in two patients (9.5%) (requiring hospitalisation and treatment interruption), grade 1 headache in one patient (4.75%) and grade 1 lower limb oedema in one patient (4.75%). There were no grade 4 or 5 adverse events.
Discussion
Several trials and a systematic review have tried to demonstrate the efficacy of BAT in patients with mCRPC, especially its effects in the re-sensitisation to hormonal therapy, but none of these trials tested BAT in heavily pretreated patients or in patients with previous use of chemotherapy. A randomised multicentre phase II trial comparing BAT versus enzalutamide in 195 men with CRPC progressed to abiraterone showed similar clinical/radiographic PFS and PSA50 in both arms, with longer biochemical PFS with BAT. Interestingly, the PSA-PFS for enzalutamide increased from 3.8 months after abiraterone to 10.9 months after BAT, and PFS2 with BAT followed by enzalutamide was significantly longer than with enzalutamide followed by BAT, proving the hypothesis that BAT can re-sensitise CRPC to subsequent antiandrogen therapy [17].
Another phase II multi cohort trial evaluated BAT in men with metastatic and non-metastatic CRPC. Two cohorts evaluated whether BAT could restore sensitivity to abiraterone and enzalutamide by treating patients who previously failed to one of these therapies with BAT and then subsequent retreatment with abiraterone or enzalutamide. In the enzalutamide cohort, a 50% decrease in PSA (PSA50) was observed in 30% of patients when treated with BAT, and 52% had a PSA response when retreated with enzalutamide. In the abiraterone cohort, PSA50 was 17% and only 16% had PSA response in retreatment [18, 19]. Finally, in the report of the cohort examining BAT as a first-line hormonal treatment for metastatic CRPC (patients not exposed to AR-targeted therapies), BAT was well tolerated and resulted in prolonged disease stabilisation, with favourable responses to subsequent second-generation AR-targeted therapies [20].
Several limitations of this analysis should be addressed. Small number of patients and a retrospective, non-randomised study limit the interpretation of data. Also, the heterogeneous population included and without a uniform indication for BAT. Even though, we observed a similar RR with enzalutamide re-challenge as in the Teply et al [18] trial and a slightly shorter PFS2 compared to Denmeade et al [17] trial. These findings support the hypothesis that BAT can re-sensitise prostate cancer cells to enzalutamide, even after several lines of treatment, and raise the question to whether BAT should be offered to these patients. mCRPC patients have limited options after becoming resistant to new hormonal agents. Usually, we use chemotherapy and/or radiopharmaceutical agents such as radium-223 or 177Lu-PSMA. These treatments should be offered to our patients because they have showed survival advantage in randomised phase 3 clinical trials, but in many instances, patients progress to all these available treatments and had no other options, been BAT the option.
Table 1. Characteristics of the patients at baseline.
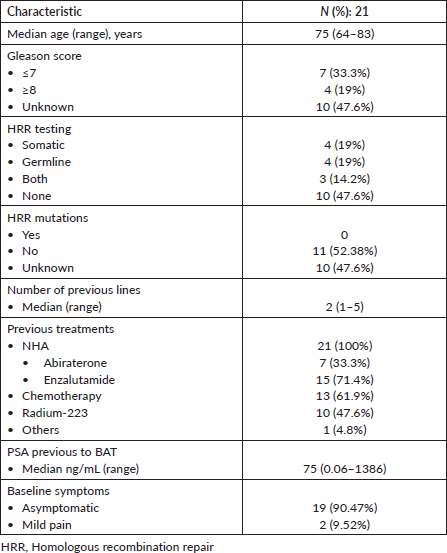
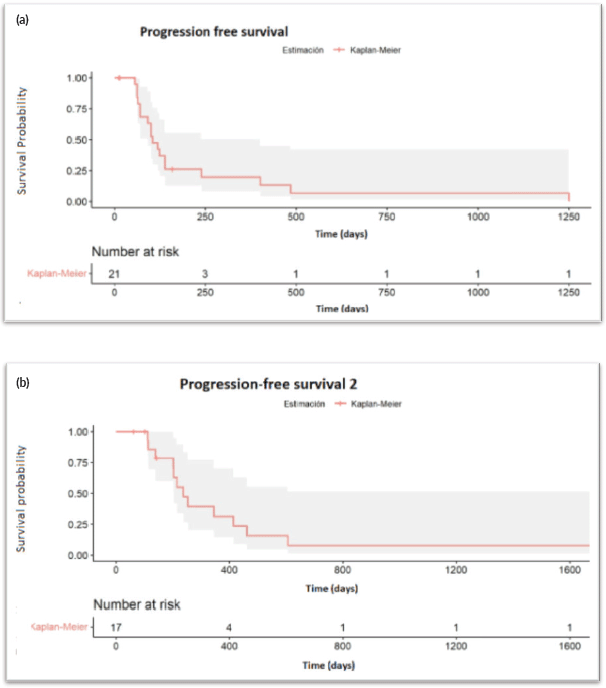
Figure 1. (a): Progression free survival. (b): Progression free survival 2.
In Latin America countries as well in lower or lower-middle income countries, access to this newer treatment is not always universal so we have to consider lines of treatment that are accessible in matter of costs and taking into account improvement of quality of life and prolonging hormonal sensitivity.
Among the advantages of using BAT, is the good tolerance to treatment, and this makes its combination with different agents such as new hormonal agents or immunotherapy feasible. To highlight, we mention the study by Markowski et al [31] where patients who received BAT and were then exposed to immunotherapy experienced 100% decreases in PSA and one patient remained in long-term remission turning this strategy into a hypothesis to be studied in future trials.
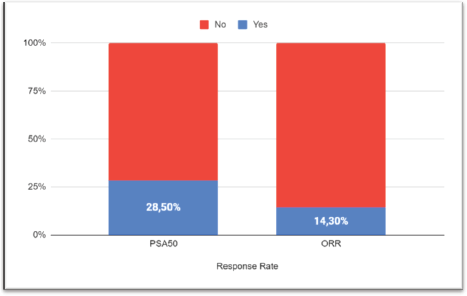
Figure 2. Response rate. PSA, Prostatic specific antigen; ORR, Overall response rate.
Table 2. Summary of cases.
Conclusion
These results were not enough to change practice in these institutions, but more physicians seem more prone to its use, especially for patients in which the effects of androgen deprivation greatly affect their quality of life, or for patients not suitable for chemotherapy. We encourage medical oncologist to discuss all these patients into a multidisciplinary tumour board.
There is still much to learn about this strategy, and more efforts should be put in prospective trials to test these findings. Until now, the only clear criterion to use BAT is in asymptomatic patients (due to the probability of worsening pain secondary to a flare effect), and perhaps a more detailed evaluation of biomarkers could help to better select patients. In addition, we believe that quality of life and cost-effectiveness studies are also necessary (even though this was not tested in our study), since it seems that these would be the greatest benefits of this therapy.
Acknowledgments
Instituto Alexander Fleming´s academic secretary.
Conflicts of interest
None of the authors have conflicts of interest to declare.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
References
1. Nelson PS (2012) Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer J Clin Oncol 30(6) 644–646 https://doi.org/10.1200/JCO.2011.39.1300
2. Huggins C and Hodges CV (2002) Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941 J Urol 167(2 Pt 2) 948–951 Discussion 952 https://doi.org/10.1016/S0022-5347(02)80307-X PMID: 11905923
3. Scher HI, Halabi S, and Tannock I, et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group J Clin Oncol 26(7) 1148–1159 https://doi.org/10.1200/JCO.2007.12.4487 PMID: 18309951 PMCID: 4010133
4. Taylor CD, Elson P, and Trump DL (1993) Importance of continued testicular suppression in hormone-refractory prostate cancer J Clin Oncol 11(11) 2167–2172 https://doi.org/10.1200/JCO.1993.11.11.2167 PMID: 8229130
5. Chen CD, Welsbie DS, and Tran C, et al (2004) Molecular determinants of resistance to antiandrogen therapy Nat Med 10(1) 33–39 https://doi.org/10.1038/nm972 PMID: 14702632
6. Watson PA, Arora VK, and Sawyers CL (2015) Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer Nat Rev Cancer 15(12) 701–711 https://doi.org/10.1038/nrc4016 PMID: 26563462 PMCID: 4771416
7. Loriot Y, Bianchini D, and Ileana E, et al (2013) Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol 24 1807–1812 https://doi.org/10.1093/annonc/mdt136 PMID: 23576708
8. Maines F, Caffo O, and Veccia A, et al (2015) Sequencing new agents after docetaxel in patients with metastatic castration-resistant prostate cancer Crit Rev Oncol Hematol 96 498–506 https://doi.org/10.1016/j.critrevonc.2015.07.013 PMID: 26318091
9. Attard G, Borre M, and Gurney H, et al (2018) Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment J Clin Oncol 36 2639–2646 https://doi.org/10.1200/JCO.2018.77.9827 PMID: 30028657 PMCID: 6118405
10. Oh WK, Cheng WY, and Miao R, et al (2018) Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting Urol Oncol 36(11) 500.e1–500.e9 https://doi.org/10.1016/j.urolonc.2018.08.002
11. De Wit R, de Bono J, and Sternberg CN, et al (2019) Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer N Engl J Med 381(26) 2506–2518 https://doi.org/10.1056/NEJMoa1911206 PMID: 31566937
12. Isaacs JT, Brennen WN, and Denmeade SR (2017) Rationale for bipolar androgen therapy (BAT) for metastatic prostate cancer Cell Cycle 16 1639</a> https://doi.org/10.1080/15384101.2017.1360645 PMID: 28820291 PMCID: 5602292
13. Kokontis JM, Hay N, and Liao S (1998) Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest Mol Endocrinol (Baltimore, MD) 12(7) 941–953 https://doi.org/10.1210/mend.12.7.0136
14. Horwich A, Hugosson J, and de Reijke T, et al (2013) Prostate cancer: ESMO consensus conference guidelines 2012 Ann Oncol 24(5) 1141–1162 https://doi.org/10.1093/annonc/mds624 PMID: 23303340
15. National Comprehensive Cancer Network (2022) Prostate Cancer (Version 3.2022) [https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf] Date accessed: 07/03/22
16. Moses M, Koksal U, and Ledet E, et al (2020) Evaluation of the genomic alterations in the androgen receptor gene during treatment with high-dose testosterone for metastatic castrate-resistant prostate cancer Oncotarget 11 15–21 https://doi.org/10.18632/oncotarget.27408 PMID: 32002120 PMCID: 6967778
17. Denmeade SR, Wang H, and Agarwal N, et al (2021) TRANSFORMER: a randomized phase II study comparing bipolar androgen therapy versus enzalutamide in asymptomatic men with castration-resistant metastatic prostate cancer J Clin Oncol 39 1371 https://doi.org/10.1200/JCO.20.02759 PMID: 33617303 PMCID: 8274807
18. Teply BA, Wang H, and Luber B, et al (2018) Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study Lancet Oncol 19 76 https://doi.org/10.1016/S1470-2045(17)30906-3 PMCID: 5875180
19. Markowski MC, Wang H, and Sullivan R, et al (2021) A multicohort open-label phase II trial of bipolar androgen therapy in men with metastatic castration-resistant prostate cancer (RESTORE): a comparison of post-abiraterone versus post-enzalutamide cohorts Eur Urol 79 692 https://doi.org/10.1016/j.eururo.2020.06.042 PMCID: 7775877
20. Sena LA, Wang H, and Lim ScM SJ, et al (2021) Bipolar androgen therapy sensitizes castration-resistant prostate cancer to subsequent androgen receptor ablative therapy Eur J Cancer 144 302 https://doi.org/10.1016/j.ejca.2020.11.043 PMID: 33383350
21. Siegel RL, Miller KD, and Jemal A (2017) Cancer statistics, 2017 CA Cancer J Clin 67 7–30 https://doi.org/10.3322/caac.21387 PMID: 28055103
22. Beer TM, Armstrong AJ, and Rathkopf DE, et al (2014) Enzalutamide in metastatic PCa before chemotherapy N Engl J Med 371 424–433 https://doi.org/10.1056/NEJMoa1405095 PMID: 24881730 PMCID: 4418931
23. Ryan CJ, Smith MR, and de Bono JS, et al (2013) Abiraterone in metastatic PCa without previous chemotherapy N Engl J Med 368 138–148 https://doi.org/10.1056/NEJMoa1209096
24. Emamekhoo H, Barata PC, and Edwin NC, et al (2018) Evaluation of response to enzalutamide consecutively after abiraterone acetate/prednisone failure in patients with metastatic castration-resistant prostate cancer Clin Genitourin Cancer 16 429–436 https://doi.org/10.1016/j.clgc.2018.08.002 PMID: 30236961
25. Smith MR, Saad F, and Rathkopf DE, et al (2017) Clinical outcomes from androgen signaling-directed therapy after treatment with abiraterone acetate and prednisone in patients with metastatic castration-resistant PCa: post hoc analysis of COU-AA-302 Eur Urol 72 10–13 https://doi.org/10.1016/j.eururo.2017.03.007 PMID: 28314611
26. de Bono JS, Chowdhury S, and Feyerabend S, et al (2018) Antitumour activity and safety of enzalutamide in patients with metastatic castration-resistant PCa previously treated with abiraterone acetate plus prednisone for >24 weeks in Europe Eur Urol 74 37–45 https://doi.org/10.1016/j.eururo.2017.07.035
27. Isaacs JT, D’Antonio JM, and Chen S, et al (2012) Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer Prostate 72 1491–1505 https://doi.org/10.1002/pros.22504 PMID: 22396319 PMCID: 3374010
28. Litvinov IV, Vander Griend DJ, and Antony L, et al (2016) Androgen receptor as a licensing factor for DNA replication in androgen-sensitive PCa cells Proc Natl Acad Sci U S A 103 15085–15090 https://doi.org/10.1073/pnas.0603057103
29. Antonarakis ES, Lu C, and Wang H, et al (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer N Engl J Med 371 1028–1038 https://doi.org/10.1056/NEJMoa1315815 PMID: 25184630 PMCID: 4201502
30. Terada N, Maughan BL, and Akamatsu S, et al (2017) Exploring the optimal sequence of abiraterone and enzalutamide in patients with chemotherapy-naïve castration-resistant PCa: the Kyoto-Baltimore Collaboration Int J Urol 24 441–448 https://doi.org/10.1111/iju.13346 PMID: 28455853
31. Markowski MC, Wang H, and De Marzo AM, et al (2022) Clinical efficacy of bipolar androgen therapy in men with metastatic castration-resistant prostate cancer and combined tumor-suppressor loss Eur Urol Open Sci 41 112–115 https://doi.org/10.1016/j.euros.2022.05.006 PMID: 35677016 PMCID: 9168525