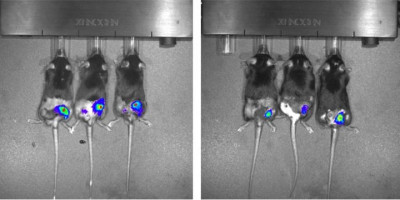
Volume 11, Issue 28 of Oncotarget features "Genetic analysis of the cooperative tumourigenic effects of targeted deletions of tumour suppressors Rb1, Trp53, Men1, and Pten in neuroendocrine tumours in mice" by Xu et, al. which reported that the authors examined whether the TSGs Rb1, Trp53, Pten, and Men1 have cooperative effects in suppressing neuroendocrine tumours in mice.
By monitoring growth and examining the histopathology of the pituitary and pancreas in these mice, the authors demonstrated that pRB had the strongest cooperative function with PTEN in suppressing Pit NETs and had strong cooperative function with Menin and TRP53, respectively, in suppressing Pit NETs and Pan NETs.
TRP53 had weak cooperative function with PTEN in suppressing pituitary lesions.
Collectively, the data indicated that pRB and PTEN pathways play significant roles in suppressing Pit NETs, while the Menin-mediated pathway plays a significant role in suppressing Pan NETs.
Understanding the molecular mechanisms of these genes and pathways on NETs will help us understand the molecular mechanisms of neuroendocrine tumourigenesis and develop effective preclinical murine models for NET therapeutics to improve clinical outcomes in humans.
Dr. Eugenia Y. Xu from Rutgers and Princeton University as well as Dr. Daniel A. Notterman from Princeton University said, "Human pituitary neuroendocrine tumours (PitNETs) are the third most common intracranial neoplasms and represent approximately 10–25% of all primary intracranial tumours."
Additionally, in rare cases, RB1 has been found with epigenetic mutations in the promoter region in Pit NETs suggesting that inactivation of the RB pathway contributes to the development of Pit NETs.
Compound mice with concomitant deletions of Men1 and Pten develop Pit NETs and Pan NETs and mice with p18–/– Pten /– mutations develop Pit NETs, suggesting that PTEN plays a role in pituitary and pancreatic islet tumourigenesis.
However, Men1 deletion mice develop pars distalis prolactinomas and Rb1 deletion mice develop pars intermedia tumours of the pituitary, suggesting that the functions of Menin and pRB may not fully overlap.
Here they investigate the question of whether Men1 and Rb1 have cooperative tumourigenic effects on NETs using tissue-specific double homozygous deletions of Men1 and Rb1 in mice.
The Oncotarget authors report that the characterisation of Pit NETs and Pan NETs with double homozygous deletions of TSGs and illustrate that pRB has the strongest cooperative function with PTEN in suppressing Pit NETs and has a strong cooperative function with Menin and TRP53, respectively, in suppressing Pit NETs and Pan NETs in mice.
The Xu/Notterman Research Team concluded in their Oncotarget Research Paper, "our data clearly demonstrate that TSGs Rb1, Pten, Men1, and Trp53 have distinct tissue specificity in neuroendocrine tumorigenesis in mouse and likely in man. The mouse models here and deletion of these TSGs in MIP-Cre mice will help further our understanding the molecular function of these TSGs and their pathways in PitNET and PanNET pathogenesis, which will help develop targeted novel therapeutic options in treating human patients."
Source: IMPACT JOURNALS LLC
The World Cancer Declaration recognises that to make major reductions in premature deaths, innovative education and training opportunities for healthcare workers in all disciplines of cancer control need to improve significantly.
ecancer plays a critical part in improving access to education for medical professionals.
Every day we help doctors, nurses, patients and their advocates to further their knowledge and improve the quality of care. Please make a donation to support our ongoing work.
Thank you for your support.